Contact:
sales@biotechnologyforums.com to feature here
sales@biotechnologyforums.com to feature here
by deeba at 10-17-2017, 10:10 PM
2 comments
Sir, please tell how should i prepare for Bioprocess engineering and process Biotechnology for Gate 2018..I am having a tough time preparing for it.
by sanjaibiotech@yahoo.com at 10-16-2017, 10:36 AM
0 comments
Department of Biotechnology, Thapar University is Organizing an International Conference on Drug Discovery: Biotechnology & Pharma at Cross Roads from 15-17th February 2018.
Conference tracks: 1. Biodiversity and Bioprospection
2. Screening Technologies for Drug Discovery
3. Natural product chemistry in Drug Development
4. Biologics, Biopharmaceuticals and Pharmacogenomics
5. Synthetic biology and Metabolomics
6. Nanotechnology and Nanomedicine
7. Interface of Academia and Industry in Drug Discovery
We are inviting speaker proposals in all tracks by 25th October 2017.
We are also inviting abstracts for poster presentation .
Registration for the conference opens on 30th October 2017.
You can please write to me at : Prof. Dr. Sanjai Saxena, Convenor, International Conference on Drug Discovery: Biotechnology & Pharma at CrossRoads, Department of Biotechnology, Thapar University, Patiala 147004, Punjab INDIA
sanjaibiotech@yahoo.com; ssaxena@thapar.edu Mobile +91 9888219815
Conference tracks: 1. Biodiversity and Bioprospection
2. Screening Technologies for Drug Discovery
3. Natural product chemistry in Drug Development
4. Biologics, Biopharmaceuticals and Pharmacogenomics
5. Synthetic biology and Metabolomics
6. Nanotechnology and Nanomedicine
7. Interface of Academia and Industry in Drug Discovery
We are inviting speaker proposals in all tracks by 25th October 2017.
We are also inviting abstracts for poster presentation .
Registration for the conference opens on 30th October 2017.
You can please write to me at : Prof. Dr. Sanjai Saxena, Convenor, International Conference on Drug Discovery: Biotechnology & Pharma at CrossRoads, Department of Biotechnology, Thapar University, Patiala 147004, Punjab INDIA
sanjaibiotech@yahoo.com; ssaxena@thapar.edu Mobile +91 9888219815
by mfiume at 10-12-2017, 02:49 AM
0 comments
Date: October 17th 2017, 7-9pm
Location: Hilton, Orlando
Price: Free
FLOW brings together thought leaders in bioinformatics to deliver a practical workshop on open, reproducible workflows. At FLOW, you will learn how to use the latest tools and standards to write, run, and scale open workflows in the cloud, including Broad's GATK4.
Refreshments will be provided.
Space is limited.
Register here
Location: Hilton, Orlando
Price: Free
FLOW brings together thought leaders in bioinformatics to deliver a practical workshop on open, reproducible workflows. At FLOW, you will learn how to use the latest tools and standards to write, run, and scale open workflows in the cloud, including Broad's GATK4.
Refreshments will be provided.
Space is limited.
Register here
by Lavkeshsharma at 10-11-2017, 04:56 AM
0 comments
by Lavkeshsharma at 10-11-2017, 04:19 AM
0 comments
Scientists discover way to reduce animal feed and food production costs by increasing a key nutrient in corn
Source:Rutgers University
Summary:Scientists have found an efficient way to enhance the nutritional value of corn -- the world's largest commodity crop -- by inserting a bacterial gene that causes it to produce a key nutrient called methionine, according to a new study.

Rutgers scientists have found an efficient way to enhance the nutritional value of corn -- the world's largest commodity crop -- by inserting a bacterial gene that causes it to produce a key nutrient called methionine, according to a new study.
The Rutgers University-New Brunswick discovery could benefit millions of people in developing countries, such as in South America and Africa, who depend on corn as a staple. It could also significantly reduce worldwide animal feed costs.
"We improved the nutritional value of corn, the largest commodity crop grown on Earth," said Thomas Leustek, study co-author and professor in the Department of Plant Biology in the School of Environmental and Biological Sciences. "Most corn is used for animal feed, but it lacks methionine -- a key amino acid -- and we found an effective way to add it."
The study, led by Jose Planta, a doctoral student at the Waksman Institute of Microbiology, was published online today in the Proceedings of the National Academy of Sciences.
Methionine, found in meat, is one of the nine essential amino acids that humans get from food, according to the National Center for Biotechnology Information. It is needed for growth and tissue repair, improves the tone and flexibility of skin and hair, and strengthens nails. The sulfur in methionine protects cells from pollutants, slows cell aging and is essential for absorbing selenium and zinc.
Every year, synthetic methionine worth several billion dollars is added to field corn seed, which lacks the substance in nature, said study senior author Joachim Messing, a professor who directs the Waksman Institute of Microbiology. The other co-author is Xiaoli Xiang of the Rutgers Department of Plant Biology and Sichuan Academy of Agricultural Sciences in China.
"It is a costly, energy-consuming process," said Messing, whose lab collaborated with Leustek's lab for this study. "Methionine is added because animals won't grow without it. In many developing countries where corn is a staple, methionine is also important for people, especially children. It's vital nutrition, like a vitamin."
Chicken feed is usually prepared as a corn-soybean mixture, and methionine is the sole essential sulfur-containing amino acid that's missing, the study says.
The Rutgers scientists inserted an E. coli bacterial gene into the corn plant's genome and grew several generations of corn. The E. coli enzyme -- 3?-phosphoadenosine-5?-phosphosulfate reductase (EcPAPR) -- spurred methionine production in just the plant's leaves instead of the entire plant to avoid the accumulation of toxic byproducts, Leustek said. As a result, methionine in corn kernels increased by 57 percent, the study says.
Then the scientists conducted a chicken feeding trial at Rutgers and showed that the genetically engineered corn was nutritious for them, Messing said.
Surprise, one important outcome was that corn plant growth was not affected
In the developed world, including the U.S., meat proteins generally have lots of methionine, Leustek said. But in the developing world, subsistence farmers grow corn for their family's consumption.
study shows that they wouldn't have to purchase methionine supplements or expensive foods that have higher methionine
Journal Reference:
Jose Planta, Xiaoli Xiang, Thomas Leustek, Joachim Messing. Engineering sulfur storage in maize seed proteins without apparent yield loss. Proceedings of the National Academy of Sciences, 2017; 201714805 DOI: 10.1073/pnas.1714805114
Source:Rutgers University
Summary:Scientists have found an efficient way to enhance the nutritional value of corn -- the world's largest commodity crop -- by inserting a bacterial gene that causes it to produce a key nutrient called methionine, according to a new study.
Rutgers scientists have found an efficient way to enhance the nutritional value of corn -- the world's largest commodity crop -- by inserting a bacterial gene that causes it to produce a key nutrient called methionine, according to a new study.
The Rutgers University-New Brunswick discovery could benefit millions of people in developing countries, such as in South America and Africa, who depend on corn as a staple. It could also significantly reduce worldwide animal feed costs.
"We improved the nutritional value of corn, the largest commodity crop grown on Earth," said Thomas Leustek, study co-author and professor in the Department of Plant Biology in the School of Environmental and Biological Sciences. "Most corn is used for animal feed, but it lacks methionine -- a key amino acid -- and we found an effective way to add it."
The study, led by Jose Planta, a doctoral student at the Waksman Institute of Microbiology, was published online today in the Proceedings of the National Academy of Sciences.
Methionine, found in meat, is one of the nine essential amino acids that humans get from food, according to the National Center for Biotechnology Information. It is needed for growth and tissue repair, improves the tone and flexibility of skin and hair, and strengthens nails. The sulfur in methionine protects cells from pollutants, slows cell aging and is essential for absorbing selenium and zinc.
Every year, synthetic methionine worth several billion dollars is added to field corn seed, which lacks the substance in nature, said study senior author Joachim Messing, a professor who directs the Waksman Institute of Microbiology. The other co-author is Xiaoli Xiang of the Rutgers Department of Plant Biology and Sichuan Academy of Agricultural Sciences in China.
"It is a costly, energy-consuming process," said Messing, whose lab collaborated with Leustek's lab for this study. "Methionine is added because animals won't grow without it. In many developing countries where corn is a staple, methionine is also important for people, especially children. It's vital nutrition, like a vitamin."
Chicken feed is usually prepared as a corn-soybean mixture, and methionine is the sole essential sulfur-containing amino acid that's missing, the study says.
The Rutgers scientists inserted an E. coli bacterial gene into the corn plant's genome and grew several generations of corn. The E. coli enzyme -- 3?-phosphoadenosine-5?-phosphosulfate reductase (EcPAPR) -- spurred methionine production in just the plant's leaves instead of the entire plant to avoid the accumulation of toxic byproducts, Leustek said. As a result, methionine in corn kernels increased by 57 percent, the study says.
Then the scientists conducted a chicken feeding trial at Rutgers and showed that the genetically engineered corn was nutritious for them, Messing said.
Surprise, one important outcome was that corn plant growth was not affected
In the developed world, including the U.S., meat proteins generally have lots of methionine, Leustek said. But in the developing world, subsistence farmers grow corn for their family's consumption.
study shows that they wouldn't have to purchase methionine supplements or expensive foods that have higher methionine
Journal Reference:
Jose Planta, Xiaoli Xiang, Thomas Leustek, Joachim Messing. Engineering sulfur storage in maize seed proteins without apparent yield loss. Proceedings of the National Academy of Sciences, 2017; 201714805 DOI: 10.1073/pnas.1714805114
by anuj at 10-11-2017, 02:25 AM
8 comments
Sir, i want to know about the internship projects. As i m pursuing B. S. C (H) biotechnology. So, i want to know tht where i can get the good interships.
by Muskan Gupta at 10-09-2017, 05:49 PM
12 comments
Can u plz tell me abt entrance exams held for MBA biotech.. n it's scop..
by Vikash at 10-07-2017, 08:29 PM
0 comments
im vikash...
hope i would learn something new and intresting.here....,?
hope i would learn something new and intresting.here....,?
by saswati mishra at 10-07-2017, 04:34 PM
0 comments
HIV GENOTYPING and role of mutation in drug resistance are different??
by saswati mishra at 10-07-2017, 03:12 PM
10 comments
Sir, can you please suggest me some recent experiment or topics related to genetics and chemical science for my internship???
by Lavkeshsharma at 10-07-2017, 04:14 AM
0 comments
by RINKI at 10-06-2017, 07:43 PM
20 comments
Is their any options for internship in research projects...???
by Lavkeshsharma at 10-06-2017, 06:52 AM
0 comments
by Lavkeshsharma at 10-06-2017, 06:38 AM
0 comments
Urgent Requirement
Designation : Bioinformatics Analyst
Qualification : Bachelor's/Master's Degree in Bioinformatics with 1-2 years experience.
Freshers in Bioinformatics with good academic record can also apply.(2016-17).
Need candidates who have done project or internship in NGS ( New Generation Sequencing )
Intersted candidates can drop their CVs to chandrakala.b@2coms.com
Or contact : 9703934675 / 040 - 33540809
Designation : Bioinformatics Analyst
Qualification : Bachelor's/Master's Degree in Bioinformatics with 1-2 years experience.
Freshers in Bioinformatics with good academic record can also apply.(2016-17).
Need candidates who have done project or internship in NGS ( New Generation Sequencing )
Intersted candidates can drop their CVs to chandrakala.b@2coms.com
Or contact : 9703934675 / 040 - 33540809
by danielleh at 10-06-2017, 06:26 AM
0 comments
Hey Guys,
Just wanted to share a cool resource for you all.
Biotech is both a high-growth and high-risk industry. In this field, success strongly relies on the science behind every concept that a company strives to make a reality. One of the main challenges in biotech is that the outcome of any long-term R&D project heavily depends on time-sensitive experiments that can sometimes cost a little fortune each.
Both public and private funding is usually limited, at least in comparison with the extremely high costs associated with R&D.
We have published a white paper on how to effectively manage the costs in your lab - “The Essential Guide to Managing Biotech Labs”. Download it for free here: http://ow.ly/fnQE30fFNfZ
Just wanted to share a cool resource for you all.
Biotech is both a high-growth and high-risk industry. In this field, success strongly relies on the science behind every concept that a company strives to make a reality. One of the main challenges in biotech is that the outcome of any long-term R&D project heavily depends on time-sensitive experiments that can sometimes cost a little fortune each.
Both public and private funding is usually limited, at least in comparison with the extremely high costs associated with R&D.
We have published a white paper on how to effectively manage the costs in your lab - “The Essential Guide to Managing Biotech Labs”. Download it for free here: http://ow.ly/fnQE30fFNfZ
by danielleh at 10-06-2017, 06:24 AM
0 comments
Hey Guys,
Just wanted to share a cool resource for you all.
Biotech is both a high-growth and high-risk industry. In this field, success strongly relies on the science behind every concept that a company strives to make a reality. One of the main challenges in biotech is that the outcome of any long-term R&D project heavily depends on time-sensitive experiments that can sometimes cost a little fortune each.
Both public and private funding is usually limited, at least in comparison with the extremely high costs associated with R&D.
We have published a white paper on how to effectively manage the costs in your lab - “The Essential Guide to Managing Biotech Labs”. Download it for free here: http://ow.ly/fnQE30fFNfZ
Just wanted to share a cool resource for you all.
Biotech is both a high-growth and high-risk industry. In this field, success strongly relies on the science behind every concept that a company strives to make a reality. One of the main challenges in biotech is that the outcome of any long-term R&D project heavily depends on time-sensitive experiments that can sometimes cost a little fortune each.
Both public and private funding is usually limited, at least in comparison with the extremely high costs associated with R&D.
We have published a white paper on how to effectively manage the costs in your lab - “The Essential Guide to Managing Biotech Labs”. Download it for free here: http://ow.ly/fnQE30fFNfZ
by Lavkeshsharma at 10-06-2017, 06:10 AM
0 comments
The Nobel prize for medicine or physiology was awarded for research on the body’s clock, which is at work in all multicellular life. But what exactly is it?

In the age of international travel , shift work and personal gadgets that stave off sleep, the award of the Nobel prize for research on the body’s clock, or circadian rhythms, could hardly be more timely.
First identified in fruit flies, the tiny molecular components of the clock are at work in all multicellular life, humans included. The internal clock is now regarded as a key feature of life on Earth, one that wired the rotation of the planet into the fabric of our cells over millions of years of evolution.
The beauty of the body’s clock is that it allows an organism to anticipate the rising and setting of the sun, rather than simply reacting to it. There is no single body clock that bangs out the hour. Instead, molecular timepieces are dotted through the different cell types, like watches in a jeweller’s, where they control great swaths of physiology from sleep patterns and body temperature to blood pressure, metabolism and the release of hormones.
Scientists knew that living organisms had internal clocks centuries before they understood what made them tick. It is hard to find a plant, bug, or animal, that does not change its behaviour in some way as day gives way to night, and night becomes day. The grandly-named French astronomer Jean-Jacques d’Ortous de Mairan performed some of the most convincing early experiments that pointed to the existence of an internal clock. In 1729, he showed that mimosa plants opened their leaves in the daytime and closed them again at night, even if they were kept in total darkness. The observation suggested the plants weren’t so much reacting to light, but somehow in tune with the day-night cycle.
Two hundred years after the death of d’Ortous de Mairan, modern scientists took their first major step towards understanding internal clocks. In 1971, the US neuroscientist Seymour Benzer and his student Ronald Konopka noticed that a batch of mutant fruit flies seemed to have faulty internal clocks. The dodgy timekeeping was traced back to mutations in a gene that was later given the name “period”.
Enter the Nobel prize winners. In 1984, Jeffrey Hall and Michael Rosbash at Brandeis University in Waltham, Massachusetts, studied the period gene and the protein the body makes from it. They showed that the protein, named PER, built up in cells overnight before being broken down in the daytime. It meant that levels of PER rose and fell over the 24-hour daily cycle.
It was far from clear what made PER go up and down like clockwork. The two scientists proposed that it was the build-up of the PER protein itself that stopped cells making more, just as wolfing down too many doughnuts dampens the desire to eat them.
In 1994, Michael Young at Rockefeller University showed that this kind of feedback loop was indeed at work. He discovered a second body clock gene that is used to make a protein called TIM. When TIM proteins come across PER proteins in cells, the two stick together, move into the nucleus, and shut the period gene down. In the late 1990s, other scientists helped piece together more genes in the clock’s mechanism, including those that help cells set the time by the light the body receives.
The body clock is more than a biological curiosity. Studies by health researchers have found evidence that disrupting circadian rhythms by doing shift work can raise the risk of cancer . It is not well understood why shift work has such health risks, but one hypothesis is that exposure to light at night suppresses levels of melatonin, a hormone that might mop up particles known as “reactive oxygen species” which cause damage to cells. In 2014, scientists found shift work and jet lag disrupt the rhythms of hundreds of genes that are normally drawn on to maintain, repair and protect the body.
Research on the body clock has helped scientists improve health. Many drugs now on the market work best when taken at the right time. The cholesterol-cutting drug Mevacor, for example, is taken at night because levels of the enzyme it targets are highest then. The same is true for low-dose aspirin used to reduce blood pressure.
In the age of international travel , shift work and personal gadgets that stave off sleep, the award of the Nobel prize for research on the body’s clock, or circadian rhythms, could hardly be more timely.
First identified in fruit flies, the tiny molecular components of the clock are at work in all multicellular life, humans included. The internal clock is now regarded as a key feature of life on Earth, one that wired the rotation of the planet into the fabric of our cells over millions of years of evolution.
The beauty of the body’s clock is that it allows an organism to anticipate the rising and setting of the sun, rather than simply reacting to it. There is no single body clock that bangs out the hour. Instead, molecular timepieces are dotted through the different cell types, like watches in a jeweller’s, where they control great swaths of physiology from sleep patterns and body temperature to blood pressure, metabolism and the release of hormones.
Scientists knew that living organisms had internal clocks centuries before they understood what made them tick. It is hard to find a plant, bug, or animal, that does not change its behaviour in some way as day gives way to night, and night becomes day. The grandly-named French astronomer Jean-Jacques d’Ortous de Mairan performed some of the most convincing early experiments that pointed to the existence of an internal clock. In 1729, he showed that mimosa plants opened their leaves in the daytime and closed them again at night, even if they were kept in total darkness. The observation suggested the plants weren’t so much reacting to light, but somehow in tune with the day-night cycle.
Two hundred years after the death of d’Ortous de Mairan, modern scientists took their first major step towards understanding internal clocks. In 1971, the US neuroscientist Seymour Benzer and his student Ronald Konopka noticed that a batch of mutant fruit flies seemed to have faulty internal clocks. The dodgy timekeeping was traced back to mutations in a gene that was later given the name “period”.
Enter the Nobel prize winners. In 1984, Jeffrey Hall and Michael Rosbash at Brandeis University in Waltham, Massachusetts, studied the period gene and the protein the body makes from it. They showed that the protein, named PER, built up in cells overnight before being broken down in the daytime. It meant that levels of PER rose and fell over the 24-hour daily cycle.
It was far from clear what made PER go up and down like clockwork. The two scientists proposed that it was the build-up of the PER protein itself that stopped cells making more, just as wolfing down too many doughnuts dampens the desire to eat them.
In 1994, Michael Young at Rockefeller University showed that this kind of feedback loop was indeed at work. He discovered a second body clock gene that is used to make a protein called TIM. When TIM proteins come across PER proteins in cells, the two stick together, move into the nucleus, and shut the period gene down. In the late 1990s, other scientists helped piece together more genes in the clock’s mechanism, including those that help cells set the time by the light the body receives.
The body clock is more than a biological curiosity. Studies by health researchers have found evidence that disrupting circadian rhythms by doing shift work can raise the risk of cancer . It is not well understood why shift work has such health risks, but one hypothesis is that exposure to light at night suppresses levels of melatonin, a hormone that might mop up particles known as “reactive oxygen species” which cause damage to cells. In 2014, scientists found shift work and jet lag disrupt the rhythms of hundreds of genes that are normally drawn on to maintain, repair and protect the body.
Research on the body clock has helped scientists improve health. Many drugs now on the market work best when taken at the right time. The cholesterol-cutting drug Mevacor, for example, is taken at night because levels of the enzyme it targets are highest then. The same is true for low-dose aspirin used to reduce blood pressure.
by Lavkeshsharma at 10-06-2017, 03:36 AM
0 comments
The 2017 chemistry laureates were recognised for developing cryo-electron microscopy. But what is it, why is it exciting and where will it take us next?
A trio of scientists share this year’s Nobel prize for chemistry: Jacques Dubochet, Joachim Frank and Richard Henderson.
Their win is for work on a technique known as cryo-electron microscopy that has allowed scientists to study biological molecules in unprecedented sharpness, not least the Zika virus and proteins thought to be involved in Alzheimer’s disease.
Being able to capture images of these biological molecules at atomic resolution not only helps scientists to understand their structures, but has opened up the possibility of exploring biological processes by stitching together images taken at different points in time.
Cryo-electron microscopy has proved valuable in helping scientists to develop drugs.It has been used in visualising the way in which antibodies can work to stop viruses being dangerous, leading to new ideas for medicines as just one example.
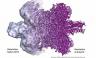
Why do we need cryo-electron microscopy?
Microscopes allow scientists to look at structures that cannot be seen with the naked eye – but when these structures are very tiny, it is no longer possible to use rays of light to do the job because their wavelengths are not short enough. Instead, beams of electrons can be used – with a technique known as transmission electron microscopy (TEM) – or scientists can employ a method known as x-ray crystallography in which x-rays are scattered as they pass through samples, creating patterns that can be analysed to reveal the structure of molecules.
The trouble is, x-ray crystallography relies on biological molecules forming ordered structures, which many fail to do, and the technique does not allow researchers to probe how molecules move.
Historically, TEM also presented difficulties. The beam itself fried the biological molecules being studied, while the technique involved the use of a vacuum which resulted in biological molecules drying out and collapsing, throwing a spanner in the works when it came to probing their structure.
This year’s chemistry laureates tackled these conundrums, enabling scientists to use TEM to image biological molecules in incredible resolution.
What did they do?
Henderson and his team, using a glucose solution to prevent molecules drying out, combined a weaker beam of electrons with images taken from many angles and mathematical approaches to build up a 3D image of a protein neatly organised within a biological membrane. It was a breakthrough moment. Henderson later succeeded in unveiling its 3D structure at atomic resolution – a first for a protein.
Meanwhile Frank developed ingenious image processing techniques to unpick TEM data and build up images of biological molecules as they are in solution, where they point in many different directions.
Dubochet came up with a sophisticated approach to prevent molecules from drying out. Henderson’s technique did not work for water-soluble biological molecules, while freezing samples resulted in the formation of ice crystals which caused damage and made the resulting images challenging to interpret.
Dubochet’s solution was to rapidly cool samples at such speed that the water molecules did not have time to adopt a regular structure. Rather, they were left pointing every which way, resulting in a glass within which biological molecules were frozen in time – in their natural shape.
What’s next?
The trio’s work, and subsequent efforts to perfect these approaches, has already led to astonishing developments.The technique of cryo-TEM has really opened up the molecular world of the cell to direct observation.
Among the processes it has made clearer is the mechanism by which DNA is copied into the single-stranded molecule RNA.
But the future is also exciting, with scientists using the technique to probe the structure of drug targets, as well as components within cells involved in sensing pain, temperature and pressure. Further improvements in resolution are also afoot.
Cryo-electron microscopy is one of those techniques so basic and important that its use spans all of biology – including understanding the human body and human disease and in designing new medicines.
A trio of scientists share this year’s Nobel prize for chemistry: Jacques Dubochet, Joachim Frank and Richard Henderson.
Their win is for work on a technique known as cryo-electron microscopy that has allowed scientists to study biological molecules in unprecedented sharpness, not least the Zika virus and proteins thought to be involved in Alzheimer’s disease.
Being able to capture images of these biological molecules at atomic resolution not only helps scientists to understand their structures, but has opened up the possibility of exploring biological processes by stitching together images taken at different points in time.
Cryo-electron microscopy has proved valuable in helping scientists to develop drugs.It has been used in visualising the way in which antibodies can work to stop viruses being dangerous, leading to new ideas for medicines as just one example.
Why do we need cryo-electron microscopy?
Microscopes allow scientists to look at structures that cannot be seen with the naked eye – but when these structures are very tiny, it is no longer possible to use rays of light to do the job because their wavelengths are not short enough. Instead, beams of electrons can be used – with a technique known as transmission electron microscopy (TEM) – or scientists can employ a method known as x-ray crystallography in which x-rays are scattered as they pass through samples, creating patterns that can be analysed to reveal the structure of molecules.
The trouble is, x-ray crystallography relies on biological molecules forming ordered structures, which many fail to do, and the technique does not allow researchers to probe how molecules move.
Historically, TEM also presented difficulties. The beam itself fried the biological molecules being studied, while the technique involved the use of a vacuum which resulted in biological molecules drying out and collapsing, throwing a spanner in the works when it came to probing their structure.
This year’s chemistry laureates tackled these conundrums, enabling scientists to use TEM to image biological molecules in incredible resolution.
What did they do?
Henderson and his team, using a glucose solution to prevent molecules drying out, combined a weaker beam of electrons with images taken from many angles and mathematical approaches to build up a 3D image of a protein neatly organised within a biological membrane. It was a breakthrough moment. Henderson later succeeded in unveiling its 3D structure at atomic resolution – a first for a protein.
Meanwhile Frank developed ingenious image processing techniques to unpick TEM data and build up images of biological molecules as they are in solution, where they point in many different directions.
Dubochet came up with a sophisticated approach to prevent molecules from drying out. Henderson’s technique did not work for water-soluble biological molecules, while freezing samples resulted in the formation of ice crystals which caused damage and made the resulting images challenging to interpret.
Dubochet’s solution was to rapidly cool samples at such speed that the water molecules did not have time to adopt a regular structure. Rather, they were left pointing every which way, resulting in a glass within which biological molecules were frozen in time – in their natural shape.
What’s next?
The trio’s work, and subsequent efforts to perfect these approaches, has already led to astonishing developments.The technique of cryo-TEM has really opened up the molecular world of the cell to direct observation.
Among the processes it has made clearer is the mechanism by which DNA is copied into the single-stranded molecule RNA.
But the future is also exciting, with scientists using the technique to probe the structure of drug targets, as well as components within cells involved in sensing pain, temperature and pressure. Further improvements in resolution are also afoot.
Cryo-electron microscopy is one of those techniques so basic and important that its use spans all of biology – including understanding the human body and human disease and in designing new medicines.
