Contact:
sales@biotechnologyforums.com to feature here
sales@biotechnologyforums.com to feature here
by Ankur at 05-31-2015, 08:51 PM
0 comments
SRM University Haryana had setup an admissions assistance booth at Career and Admission Fair held on 31 May 2015 at Pragati Maidan, New Delhi. Here Ms. Sumati gave us pretty good information about their college. I hope to hear from Dr. Samuel Raj (HOD Biotechnology) who will help us further improve this article.
I want to add information like Placement for biotechnology students and other useful details which will help class 12th students make up their mind regarding this college.







Note from Administrator:
We request college admin staff or currently enrolled students of this college to help us keep this information up to date so that our forum members are presented with the most accurate information.
I want to add information like Placement for biotechnology students and other useful details which will help class 12th students make up their mind regarding this college.
Note from Administrator:
We request college admin staff or currently enrolled students of this college to help us keep this information up to date so that our forum members are presented with the most accurate information.
by Ankur at 05-31-2015, 07:42 PM
0 comments
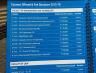
This information about LPU was provided to me during my Career and Admission Fair held on 31 May 2015 at Pragati Maidan, New Delhi. Hope this first hand information should be helpful for students looking to take admission in Lovely Professional University.
LPU is a good but an expensive university, but if you are able to avail scholarships then it becomes fairly affordable.
Placement information
I was told that in year
2013-2014 academic session (biotechnology only)
- Eligible students - 28 ( based on academic performance at LPU)
( Note: I was told that if any student decides to go for higher studies or wants to pursue career of his own, they are not counted here, therefore number may look low. LPU does not want companies to give offer letters to students and then their students do not join them. Therefore they are not counted as eligible students from the get go.)
- Placed: 19
- Max offer 3 Lakhs
- Average offer - 2.17 Lakhs
2014-2015 academic session (biotechnology only)
- Eligible students - 29
- Placed: 20
- Max offer 6.5 Lakhs
- Average offer - 2.8 Lakhs
Biotechnology Programmes After 12th
- B.Tech. Duration: 4 years (Semester System) Details here: http://www.lpu.in/program/btech.php
- B.Sc. Biotechnology (Hons.) - Duration: 3 yrs. (Semester System) View Details
- B.Sc. Biotechnology - Duration: 3 yrs (Semester System) View Details
- Integrated B.Sc. – M.Sc. Biotechnology - Duration: 4 years (Semester System)
- M. Tech. Biotechnology (BT) [Full Time] Duration: 2 yrs(Semester System) http://www.lpu.in/program/mtech-full-tim...nology.php
Here is the 2015 Admissions brochure / prospectus 12th (10+2) that was provided to me during my visit. Hope its helpful ..









Hostel room, food and Laundry charges

Note from Administrator:
We request college admin staff or currently enrolled students of this college to help us keep this information up to date so that our forum members are presented with the most accurate information.
LPU is a good but an expensive university, but if you are able to avail scholarships then it becomes fairly affordable.
Placement information
I was told that in year
2013-2014 academic session (biotechnology only)
- Eligible students - 28 ( based on academic performance at LPU)
( Note: I was told that if any student decides to go for higher studies or wants to pursue career of his own, they are not counted here, therefore number may look low. LPU does not want companies to give offer letters to students and then their students do not join them. Therefore they are not counted as eligible students from the get go.)
- Placed: 19
- Max offer 3 Lakhs
- Average offer - 2.17 Lakhs
2014-2015 academic session (biotechnology only)
- Eligible students - 29
- Placed: 20
- Max offer 6.5 Lakhs
- Average offer - 2.8 Lakhs
Biotechnology Programmes After 12th
- B.Tech. Duration: 4 years (Semester System) Details here: http://www.lpu.in/program/btech.php
- B.Sc. Biotechnology (Hons.) - Duration: 3 yrs. (Semester System) View Details
- B.Sc. Biotechnology - Duration: 3 yrs (Semester System) View Details
- Integrated B.Sc. – M.Sc. Biotechnology - Duration: 4 years (Semester System)
- M. Tech. Biotechnology (BT) [Full Time] Duration: 2 yrs(Semester System) http://www.lpu.in/program/mtech-full-tim...nology.php
Here is the 2015 Admissions brochure / prospectus 12th (10+2) that was provided to me during my visit. Hope its helpful ..
Hostel room, food and Laundry charges
Note from Administrator:
We request college admin staff or currently enrolled students of this college to help us keep this information up to date so that our forum members are presented with the most accurate information.
by Miles E. Drake at 05-24-2015, 10:11 PM
1 comments
Multiple sclerosis (MS) is a chronic inflammatory disease of the myelin sheath around nerve axons in the central nervous system, either because of attack on the myelin by the immune system or failure of the myelin-producing cells. It has had various names in the 150 years since its description, including sclerose en plaques, disseminated sclerosis and encephalomyelitis disseminate, reflecting its tendency to affect different places in the nervous system at different points in time and the plaque-like areas of scarring and atrophy that these attacks cause in the brain and spinal cord.
MS probably existed in ancient times, but clinical descriptions cannot be identified in ancient medical texts in the way that cases of epilepsy and dementia can. This may be because the complex and variable symptoms and signs of the disorder often resulted in its being diagnosed as something else, as has happened not infrequently in more recent times; it is also possible that the disease’s predilection for the temperate zones and northern climes made it less common in the African, Near Eastern and Southern European locations in which the earliest medical texts tended to be written [2]. One of the earliest clinical descriptions of what is probably MS was written around 1200 in Iceland about Halldora, a young woman with episodic visual loss and paralysis that remitted after prayers to the saints. A Dutch nun, Saint Lidwina of Schiedam, had intermittent paroxysmal pain, visual loss and leg weakness after falling while skating on a frozen canal at age 16; she died at 53 in 1433 after multiple such attacks, and became the patron saint of the chronically ill and of figure skaters. Reports of waxing and waning neurological signs and symptoms with a predilection for the visual pathways and the spinal cord came predominantly from northern Europe, and have led to the suggestion of a "Viking gene" that is responsible for the disease [3].
The clinical features and distinctive pathology of sclerose en plaque were delineated by Charcot in 1868. These features had been observed before, and also described in the writings of at least one patient, but Charcot’s contribution, as with so many other neurological diseases, was to synthesize them and to differentiate them from the signs and symptoms of other disorders and establish criteria for diagnosis of the disease. Robert Carswell in England and Jean Cruveilher in France had described the signs and symptoms of the disease and found scarring and atrophy of the spinal cord in some patients during the 1830s, but did not recognize this as a distinct disease. The Swiss anatomist Georg Rindfleisch showed in 1863 that inflammatory lesions of the spinal cord were often distributed around blood vessels, but did not draw conclusions from this. Charcot demonstrated the cardinal symptoms that became part of a diagnostic triad that bears his name – nystagmus, intention tremor and scanning or telegraphic speech – and also found that patients had slowing of cognition and enfeeblement of memory, which was not generally agreed upon as a manifestation of MS for another century[4].
Several literary descriptions of MS are in retrospect evident in the 19th century. Augustus Frederick d’Este, grandson of King George III, kept a detailed diary from 1822 until 1846 that described transient monocular visual loss, leg weakness, hand clumsiness, dizziness, numbness, unsteadiness requiring the use of a wheelchair, urinary incontinence and impotence consistent with MS. A later diarist, Bruce Frederick Cummings, wrote under the pen name of W.N.P. Barbellion an account of progressive neurologic deficit consistent with what is today called chronic progressive MS, that resulted in his death at age 31 in 1919 [5].
In the decades after Charcot’s description, salient features of MS were delineated by Walter Moxen in Britain and Edward Seguin in the United States: female predominance, generally non-inherited character and relapsing and remitting course. The causes of the disorder remained obscure, in part because the myelin sheath around nerve axons was not known until reported by Louis-Antoine Ranvier in 1878, and it was not until 1916 that James Dawson described the microscopic evidence of inflammation in myelin that is the hallmark of the MS plaque. Oligodendrocytes, the glial cells that produce myelin, were identified in 1928, and the role of the myelin sheath in rapid axonal transmission of nerve impulses (saltatory conduction) began to be understood at that time [6].
It was widely believed at that time that some unidentified toxin caused MS, and the occasional occurrence of MS-like "paralytic accidents" after rabies vaccinations, suggested that viral infection might play a role. An animal model (experimental allergic encephalomyelitis) was developed in the 1930s by Thomas Rivers and colleagues at Rockefeller University, and it was shown that immune cells and not viruses were responsible for the inflammatory attacks [7]. Seven decades of subsequent research have identified multiple components of the immune system that are responsible for autoimmune diseases: T-cell lymphocytes, antibodies, complement and chemokines [8].
In subsequent decades a number of other theories were proposed as to the cause and mechanisms of MS, such as chronic circulatory insufficiency due to arterial or venous disease or depletion of the gut biome causing susceptibility to infection or altered immune function. Various treatments including cerebral vasodilators, surgical procedures to improve venous drainage, radiation therapy and probiotics have been attempted. The major therapeutic advances, however, have involved modulation of the immune system with corticosteroids, plasmapheresis, interferon and its derivatives and the antineoplastic drug mitoxantrone. Recent attention has been focused on antibodies raised against attacking immune cells, neuroprotective agents and drugs that improve the ability of the damaged myelin sheath to conduct nerve impulses [9].
CLINICAL FEATURES
![[Image: 640px-Symptoms_of_multiple_sclerosis.svg.png]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/bf/Symptoms_of_multiple_sclerosis.svg/640px-Symptoms_of_multiple_sclerosis.svg.png)
Sensory loss or disturbance (paresthesias) are often the earliest complaint, as is twitching of the facial muscles (myokimia). Eye symptoms, particularly diplopia, occur in about a third of patients, pain in about half of patients during their clinical courses and constitutional symptoms, especially dizziness and fatigue, are reported by 70 per cent of patients. Heat intolerance, depression and less frequently euphoria and subjectice difficulties with memory or attention are commonly reported. Charcot described a symptomatic triad of ataxia due to cerebellar involvement, nystagmus and tremor which bears his name. Facial weakness, more often facial pain due to trigeminal neuralgia and spinal cord symptoms involving weakness, bowel and bladder disturbance, sexual dysfunction or transverse myelitis are also common [11].
Optic neuritis is the first clinical event in 20 per cent of patients and 40 per cent eventually experience it. Fatigue is reported by as many as 75 per cent of patients, and is the leading cause of disability in 50 to 60 per cent. Spasticity is nearly universal, and the increased muscle tone and greater difficulty of performing movements and activities contributes to fatigue. Forty to 70 per cent of MS patients have cognitive difficulties, chiefly affecting memory but also involving visual perception and executive function, that also adversely affect quality of life and contribute to disability. About half of patients have significant pain, which is mainly neuropathic in character and related to the demyelinating lesions, but can also over time arise from spasticity, abnormal posture or impaired balance. Urinary symptoms can involve failure of the bladder to store urine properly, resulting in frequency, urgency or incontinence, or failure to empty normally, causing urinary retention and infection. Constipation is the most common intestinal complaint, due either to neurogenic bowel dysfunction from spinal lesions or to bowel immobility. Aphasia and other language disturbances or seizures are rare, reported by less than 5 per cent of patients [12].
The physical and neurological examination is often abnormal in MS and may change from examination to examination, not always in conjunction with clinical symptoms. The most common abnormalities are localized weakness, generally with spasticity of the involved limbs; focal sensory impairment, particularly of posterior column modalities (vibration and proprioception); slowing and decreased coördination of limb movements and an ataxic wide-based gait ("spastic ataxia"). Optic neuritis commonly involves unilateral visual loss or impairment, sometimes painful, but is usually retrobulbar in location and may cause only pallor of the optic disc on fundoscopic examination ("the patient sees nothing and the doctor sees nothing"). Eye movements are frequently abnormal, most commonly inability to move one eye laterally due to abducens nerve palsy or bilateral internuclear ophthalmoplegia from involvement of the medial longitudinal fasciculus in the brain stem, resulting in inability to move the eye on the side of the lesion to the midline (adduction) along with nystagmus in the other (abducting) eye [13].
MS is clinically classified on the basis of relapses and remissions or their absence. Relapsing-remitting MS (RRMS) is the classical form, and accounts for about 85 per cent of cases. Studies with serial MRI imaging indicate that asymptomatic demyelinating episodes can occur in between clinical relapses. About half of patients with RRMS convert to a secondary progressive form (SPMS) after 10-15 years of disease; attacks no longer remit and there is progressive accumulation of disability. Primary progressive MS (PPMS) is progressive without remissions from the start, and represents about 10 per cent of cases; 5 per cent of patients have a progressive course with periodic superimposed relapses (PRMS). There are also milder variants: the clinically isolated syndrome (CIS) , also called "possible MS" involves a single MS-like attack and some patients have mild and infrequent attacks with complete resolution of symptoms and little or no accumulation of deficit over 15 to 20 years of disease (Benign MS) [14].
The severity and progression of MS are measured by disability scales, chiefly the Kurtzke Disability Status Scale, developed in 1955 and now considerably expanded (EDSS). Grades from 0 to 4 are determined by functional system (FS) scores for pyramidal (motor), cerebellar, brain stem, sensory, bowel and bladder and visual status as well as other aspects of neurologic function, particularly cognition. These yield scores ranging in increments of 0.5 from a score of 0 for normal neurologic examination and cerebral function to a score of 10 for death from MS. Another simple measure of disability is the Ambulation Index, which is the length of time required to walk 25 feet [15].
EPIDEMIOLOGY
MS is the most common autoimmune disorder involving the central nervous system, and affected 30 people per 100,000 with 2.5 new cases per 100,000 and 18,000 deaths in 2010. There are marked geographic and climate differences in prevalence, with 0.5 cases per 100,000 in Africa but 9 per 100,000 in the Americas and 80 per 100,000 in Europe generally, and 200 per 100,000 in northern European populations. Its onset is most commonly in the 20s and 30s, and at that age women predominate over men by about 2 to 1 and most cases are the classical RRMS. In cases with onset after age 50, incidence is about equal in women and men and PPMS is the most common form [16]. Individuals born in areas with high MS incidence who move to areas with low incidence before age 15 acquire the new region’s risk for MS, but if they migrate after age 15 they keep the MS risk of the area in which they were born. Many potential risk factors for MS have been scrutinized, generally with equivocal or results; there is an increased risk with smoking, and gout is less frequent among MS patients than would be expected while serum uric acid levels are lower than in individuals without MS [17].
GENETICS
Inheritance does not play a strong part in the acquisition of MS. The concordance rate between monozygotic twins is only 20 to 30 per cent, and the risk of developing the disorder is about 7 times greater for first-degree relatives like children and siblings than for the general population but the excess risk in families over a lifetime is only about 5 per cent. This is consistent with minimal genetic predisposition and greater effect from some environmental exposure, as is the occurrence of conjugal MS in husbands and wives who do not have any MS in their own biological families. Rather than gene mutations associated with the disease, it has been suggested that variants of genes (polymorphisms) may cause different degrees of gene expression in different people, and if genes regulating immune activity are involved, then some of those people may have exaggerated expression of a proinflammatory gene that results in untoward immune reactions and autoimmune disorders. A polymorphism may result in greater likelihood of developing MS or having a more severe or unremittent form of the disease if exposed to a trigger for the development of symptoms, while other polymorphisms might confer some degree of protection against the disorder.
Polymorphisms of the HLA genes located on chromosome 6 (MHC or major histocompatibility complex) have been linked to susceptibility to autoimmune diseases including MS, type 1 diabetes and systemic lupus erythematosus. Increased MS risk has been associated with MHC alleles DR15 and DQ6, while other HLA alleles have had an apparent protective effect against developing MS (HLA-C554 and HLA-DRB1*11. About half of the modest genetic predisposition to MS is apparently due to these HLA-MHC genes, while the rest is due to 12 or more genes in other locations, polymorphisms of which may increase the risk of developing MS [18].
CAUSES
If genetic factors predispose certain individuals to an excessive immune reaction and the development of autoimmune disorders, or in some cases genetic protection against immune overreaction is absent, there must still be some event or agent that triggers the immune process. Viral infections have been suggested to activate self-reactive T-cells that may react against myelin, and chronic virus infection with periodic reactivation could produce remissions and relapses of disease. Several viruses have been proposed as MS triggers, initially measles and canine distemper and most recently Epstein-Barr virus (EBV). MS patients have a high incidence of serum antibodies against EBV and EBV antigens are often expressed in MS plaques, but most people with EBV infection do not develop MS and many patients with MS do not have evidence of EBV infection [19].
The geographic predilection of MS has raised the question of environmental causes in northern latitudes. If there is an environmental factor involved, it must exert its effect early as moving to a new location after age 15 causes people to incur the MS risk of their new domicile thereafter; in addition, some ethnic groups in regions farther from the Equator that have high MS incidences do not themselves often develop MS, such as Canadian Inuits, American Eskimos, the Sami or Lapps of Finland and the Maori of New Zealand. There is also some evidence in recent studies that the north-south differential in MS incidence is decreasing [20]. Vitamin D levels have been proposed as a causative environmental factor and vitamin D has a role in regulating immune response by decreasing the production of proinflammatory cytokines and increasing the production of anti-inflammatory cytokines. High circulating levels of vitamin D appear to be associated with a reduced risk of MS, and while vitamin D might be deficient in some northern areas that have less sunlight and higher MS incidence, studies suggest that Norway, where the traditional diet is very high in vitamin D, has a lower incidence of MS than its Scandinavian neighbors [21].
Hepatitis vaccination and venous insufficiency are recent suggestions for possible causative factors in MS that have not yet gained wide acceptance. Anecdotal reports that MS was more frequent after hepatitis vaccination prompted investigation by the Centers for Disease Control and Prevention (CDC), but an increased incidence of the disease among hepatitis-vaccinated individuals was not confirmed [22]. Several studies have suggested that venous stenosis and insufficient cerebral venous drainage can lead to venous stasis, iron deposition in the brain and the triggering of an immune reaction causing MS. Internal jugular vein-azygos vein angioplasty was reported to alleviate MS symptoms, but this study has not yet been replicated [23].
DIAGNOSIS
MS has historically been diagnosed by history and neurological findings. Current criteria specify that there have been two or more objectively evident attacks at different times, two separate episodes with clinical evidence from one and MRI abnormality ascribable to the other, one objectively-verifiable clinical attack with MRI evidence of two or more others in the past, a single episode of neurological deficit (clinically isolated syndrome) followed by a later episode or MRI abnormality or insidious progression of neurological deficit over a year accompanied by one brain lesion on MRI, two spinal cord lesions or abnormal cerebrospinal fluid studies [14].
MRI imaging is highly sensitive to the lesions of MS, being positive for 90 to 95 per cent of brain lesions and 75 per cent of spinal cord lesions, more in older than in younger patients. T2-weighted images show "bright objects" caused by edema associated with lesions, while T1-weighted images detect "black holes" from axonal death and resultant cerebral atrophy. Functional MRI and Magnetic Resonance spectroscopy may detect changes in patients with slight or absent changes on structural brain MRI or minimal clinical findings despite substantial MRI lesions. The current focus in MS neuroimaging is on correlation with therapeutic response [24].
Lumbar puncture was once routinely done and remains the only objective test for central nervous system inflammation; it is often helpful in patients with possible MS. Oligoclonal bands are present in 90 to 95 per cent of patients, and 70 to 90 per cent of those with MS will have intrathecal production of IgG [25]. Standard EEG is variably and nonspecifically abnormal in MS, but computer-assisted quantitative EEG shows differences between MS with benign prognosis and relapsing-remitting forms [26]. Evoked potentials to visual, auditory and somatosensory stimulation extracted from the EEG by signal averaging have been largely superceded by MRI, but may show separate lesions and predict MS course and effect of therapy [27].
Cerebral angiography is rarely used to differentiate MS and cerebral vasculitis. Ultrasound examination has been suggested for measurement of ventricular size in the evaluation of cognitive dysfunction in MS. Laboratory studies are appropriate to exclude differential possibilities such as Lyme disease, syphilis, thyroid disease, vitamin B12 deficiency and Wilson’s disease; Devic’s disease or neuromyelitis optica, which can more closely resemble MS, is associated with antibodies to the nerve membrane water channel aquaporin 4 [28].
TREATMENT
For about a century there was no effective treatment for MS. In the last 25 years there have been substantial advances in its management, due in large part to biotechnology advances in the areas of neuroimaging, drug development, genetics and immunology. MS therapy now involves disease-modifying or immunomodulatory treatments to reduce the frequency and severity of relapses and slow progression of the disease, and symptomatic management of the effects of demyelination or neurological deficit. With one exception, disease-modifying drugs are approved only for the relapsing form of MS. Symptomatic management focuses on spasticity, bowel and bladder disturbances, effects of paralysis and immobility, alleviation of pain and amelioration if possible of cognitive and behavioral problems. There is evidence that prompt treatment of clinically isolated symptoms reduces the conversion of possible to probable or definite MS by about 40 per cent [29].
Acute relapses have historically been treated with corticosteroids, first ACTH and more recently methylprednisolone. This alleviates acute symptoms but may not change overall disease progression, and it is not clear that it works for optic neuritis. Plasmapheresis is considered probably effective as second-line treatment when steroids do not work or cannot be used [30].
Ten immunomodulatory drugs are now approved for relapsing MS in the United States. Three (fingolimod, teriflunomide and dimethyl fumarate) are taken orally, interferon-beta-1a is administered intramuscularly, pegylated interferon-beta-1a (mixed with PEG or polyethylene glycol for longer duration of action), interferon-beta-1b and glatirimer acetate are given subcutaneously and natalizumab and mitoxantrone require intravenous infusion [31].
Interferon-beta-1b (Betaseron) was the first agent to be approved in 1993, and decreased the frequency of relapses by about a third, significantly reduced the acquisition of MS lesions and brought about a 10 per cent reduction in the incidence of disease progression. Side effects include asthenia, fatigue, depression, increased muscle tone, skin reactions at the injection site, leukopenia and increase liver enzymes, myasthenia and most commonly, flu-like symptoms.
Intramuscular interferon-beta-1a (Avonex) decreased the annual exacerbation rate by 29 per cent, reduced disease progression from 35 per cent in the placebo group to 21 per cent in the treatment group and significantly decreased the number and volume of MRI enhancing lesions. The subcutaneous form (Rebif) reduced relapses by 27 (low dose) and 33 (high dose) percent, and was shown in other studies to reduce cumulative disability and the burden of MRI lesions with the higher dose. Flu-like symptoms were less than with interferon-beta-1b but injection site reactions, white blood cell abnormalities and liver disorders were more common. The subcutaneous preparation had fewer flu-like reactions, but skin reactions and abnormalities of liver function and white blood cells were more common. Both forms of interferon-beta-1a were associated with neutralizing antibodies which lessened drug effect, and this was more common with the subcutaneous form. Interferon-beta-1a admixed with PolyEthylene Glycol (PEGylated and called Plegridy) can be self-administered subcutaneously every 2 weeks, and reduced the relapse rate by 36 per cent, decreased disability progression by 38 per cent and lessened the appearance of new MRI lesions by 67 to 85 per cent as compared to placebo. Interferon-beta-1may be problematic in patients with uncontrolled depression, and consideration of glatirimer is recommended instead.
Glatirimer acetate (Copaxone) is a synthetic polypeptide that produced a 29 per cent reduction in relapse rate over 2 years and may have slightly reduced disability; it was therefore approved by the Food and Drug Administration for relapse prevention but not for slowing disability progression. A higher dose approved in 2014 may be more effective, and can be administered subcutaneously 3 times a week rather than once daily.
Natalizumab (Tysabri) is a monoclonal antibody, hence the "mab" in its name, that binds to the adhesion molecule alpha-4 integrin and prevents it from adhering to receptors and presumably from initiating an immune response. It is raised in mice and can therefore be immunogenic itself, so it is altered and "humanized" for monthly intravenous infusion. Natalizumab reduced relapses by 68 per cent and disease progression by 42 per cent over 2 years, but was also associated with fatal cases of the opportunistic viral infection progressive multifocal leukoencephalopathy and was therefore withdrawn in 2005. It was reintroduced in 2006 for patients with very active relapsing disease or no response to interferon or glatirimer. It may also work for primary and secondary progressive MS, but is available only through a restricted distribution system.
Fingolimod (Gilenya) is derived from miryocin, produced by a fungus related to several used in Chinese and Tibetan medicine. Fingolimod is an analogue of the cell membrane lipid sphingosine, and through sphingosine-1-phosphate receptors causes lymphocytes to remain in lymph nodes so they cannot initiate an immune attack; it may also stimulate the repair and replacement of glial cells, perhaps including the ones which synthesize myelin. It was the first MS drug that can be taken orally, and in daily administration significantly reduced relapses and delayed the acquisition of disability. Reduced lymphocyte count and resultant infection, macular edema, skin cancer and bradycardia and hypotension have occurred, the latter sufficiently severe that the first doses should be taken under medical observation and an ECG obtained before and after. The drug is now contraindicated in patients with cardiovascular disease.
Teriflunomide (Aubagio) inhibits the rapid division of cells, and may therefore retard the activation of T-cells to start an immune response. It produced a 31 per cent reduction in relapse rate and minimally decreased the progression of disability, and significantly reduced the development of definite MS in patients with clinically isolated syndromes. Decreased white blood cell count and increased blood pressure may occur along with flu-like symptoms and other nonspecific effects; liver toxicity, teratogenesis, peripheral neuropathy, acute renal failure, immunosuppression with infection and particularly activation of latent tuberculosis, skin hypersensitivity reactions, interstitial lung disease, hyperkalemia and hypophosphatemia. It is contraindicated in patients with liver disease, women of childbearing age who are not sterilized or using contraceptives and those taking its parent compound leflunomide (Arava) for arthritis.
Dimethyl fumarate (Tecfidera) was approved for oral treatment of relapsing MS because it decreased the proportion of patients who relapsed by half, decreased the annual rate of relapse by 53 per cent and caused a 38 per cent reduction in progression of disability over 2 years as compared to placebo. The appearance of MRI lesions was also significantly reduced compared to placebo. The drug activates the nuclear factor (erythroid-derived 2)-like 2 or Nrf2 pathway that regulates the synthesis of antioxidant proteins that protect against cell injury and inflammation, and may therefore have a neuroprotective effect. It is also an agonist of the nicotinic acid receptor, which may be helpful for oxidative cell stress and could account for the side effects of flushing, gastrointestinal upset, diarrhea and nausea. White blood cell count and liver function tests need to be monitored also.
The most recent addition to the disease-modifying armamentarium is alemtuzumab (Lemtrada), developed for chemotherapy of leukemia and lymphoma and also used as an immunosuppressant in preparation for kidney, islet cell and bone marrow transplantation. It is a recombinant monoclonal antibody against the CD52 lymphocyte antigen, and cells to which it binds are targeted for destruction. As a result, the risk of severe autoimmune reactions is significant, but the drug was significantly more effective than interferon-beta-1a or 1b in patients new to treatment or relapsing during treatment. In a later study, a quarter of the patients treated with alemtuzumab became free of disease activity (no relapses, no accumulation of disability, no new MRI lesions) while none of the interferon-treated patients did.
Mitoxantrone (Novantrone) is approved for secondary progressive MS, relapsing MS that is worsening and for progressive relapsing disease. It is an immunosuppressive agent used for lymphocytic leukemia, lymphoma and breast cancer, and has been given "black box" warnings by the FDA on account of cardiac toxicity and risk of developing acute myelogenous leukemia. Some of the same adverse effects, along with hemorrhagic cystitis, are associated with cyclophosphamide (Cytoxan) therapy, but this immunosuppressive agent is effective in inducing remission of aggressive relapsing or secondary progressive MS, followed by maintenance therapy with glatirimer. No therapy is presently approved for primary progressive MS.
Pharmacological treatment, physical and occupational therapy, speech therapy and cognitive-behavioral psychotherapy have been shown to be effective for the management of symptoms and disability. The areas in which symptomatic therapy is appropriate include visual impairment from optic neuritis, fatigue, spaticity, bowel and bladder dysfunction, cognitive decline, depression and less frequently agitation, paroxysmal disturbances and particularly pain, tremor, heat intolerance and sexual dysfunction32. If not treated in one way or another, about a third of patients will have permanent physical disability after 20 to 25 years of disease. Five to 10 per cent will manifest apparently benign disease, with little or no disability after a long course, but these patients may show cognitive deterioration over time. Patients with primary progressive disease or spinal cord lesions from MS will have the worst prognosis, particularly males. Life expectancy is in general only modestly shortened, and death is caused by secondary infectious, pulmonary or renal complications in about two-thirds of patients. Although MS remains a significant long-term treatment and management problem, the accelerating pace of diagnostic and therapeutic development, due in large part to advances in genetic and drug development technologies, has improved its outlook over the past 25 years.
REFERENCES
1. Compston A, Coles A (2008). Multiple sclerosis. Lancet, 372(9648): 1502-1517.
2. Maeder R (1979). Does the history of multiple sclerosis go back as far as the 14th century? Acta Neurol Scand, 60 (3): 189–92.
3. Holmøy T (2006). A Norse contribution to the history of neurological diseases. Eur. Neurol. 55 (1): 57–8.
4. Murray TJ (2005). Multiple Sclerosis: The History of a Disease. New York, Demos Press.
5. Orrell RW (2005). Multiple Sclerosis: The history of a disease (review). J Roy Soc Med, 98(6): 289.
6. Piccolino M (2003). Nerves, alcohol and drugs, the Adrian-Kato controversy on nervous conduction: deep insights from a "wrong" experiment? Brain Res Rev, 43(3): 257-265.
7. Van Epps HL (2005). Thomas Rivers and the EAE model. J Exp Med, 202(1): 4.
8. Steinman L (2003). Optic neuritis, a new variant of experimental encephalomyelitis, a durable model for all seasons, now in its seventieth year. J Exp Med, 197(9): 1065-1071.
9. Sontheimer H (2015). Multiple sclerosis. In, Diseases of the Nervous System. Waltham MA; Academic Press. New diagnostic criteria for multiple sclerosis: guidelines for research protocols.
10. Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtelotte WW (1983). New diagnostic criteria for multiple sclerosis: guideline for research protocols. Ann Neurol, 13(3): 227-231.Ann Neurol. 1983; 13(3):227-31.
11. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sanberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001). Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol, 50(1):121-127.
12. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sanberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005). Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol, 58(6): 840-846.
13. National Clinical Guideline Center (2014). Management of Multiple Sclerosis in Primary and Secondary Care (NICE Clinical Guidelines, No. 186). London: National Institute for Health and Care Excellence (UK).
14. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA. Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L. Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol, 69(2):292-302.
15. Kurtzke JF (1983). Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology, 33(11):1444-52.
16. Milo R, Kahana E (2010). Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun Rev, 9(5): A387-A394.
17. Ascherio A, Munger KL (2007). Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol, 61(6): 504-513
18. Baranzini SE (2011). Revealing the genetic basis of multiple sclerosis: are we there yet? Curr Opin Genet Devel, 21(3): 317-324.
19. Ascherio A, Munger KL (2007). Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol, 61(4): 288-299.
20. Salvetti M, Giovannoni G, Aloisi F (2009). Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol, 22(3):201-6.
21. Kampman MT, Brustad M (2008). Vitamin D: a candidate for the environmental effect in multiple sclerosis - observations from Norway. Neuroepidemiology, 30(3):140-6.
22. Centers for Disease Control and Prevention (2010). FAQs about Hepatitis B Vaccine (Hep B) and Multiple Sclerosis. Bethesda, MD. US Department of Health and Human Services.
23. Lapaucis A, Lillie E, Dueck A, Straus S, Perrier L, Burton JM, Aviv R, Thorpe K, Feasby T, Spears J (2011). Association between chronic cerebrospinal venous insufficiency and multiple sclerosis: a meta-analysis. CMAJ, 183(16):E1203-12.
24. Sicotte NL (2011). Neuroimaging in multiple sclerosis: neurotherapeutic implications. Neurotherapeutics, 8(11): 54-62. therapeutics. 2011 Jan;8(1):54-62.
25. Dobson R, Ramagopalan S, Davis A, Giovannoni G (2013). Oligoclonal bands in multiple sclerosis and clinically isolated syndromes. Cerebrospinal Fluid Oligoclonal Bands in Multiple Sclerosis and Clinically Isolated Syndromes. A meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiat, 84(8): 909-914.
26. Vazquez-Marrufo M, Gonzalea-Rosa JJ, Vaquero E, Duque P, Borges M, Gomez C, Izquierdo G (2008). Quantitative electroencephalography reveals different physiological profiles between benign and remitting-relapsing multiple sclerosis patients. Quantitative electroencephalography reveals different physiological profiles between benign and remitting-relapsing multiple sclerosis patients. BMC Neurol, 8:44.
27. Margaritella N, Mendozzi L, Garegnani M, Nemni R, Colicino E, Gilardi E, Pugnetti L (2012). Exploring the predictive value of the evoked potentials score in MS within an appropriate patient population. A hint for an early identification of benign MS? BMC Neurology, 12: 80.
28. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005). IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med, 202(4): 473-477.
29. Freedman MS (2013). Teriflunomide in relapsing multiple sclerosis: therapeutic utility. Ther Adv Chron Dis, 4(5): 192-205.
30. Cortese I, Chaudhry V, So YT, Cornblath DR, Rae-Grant A (2011). Evidence-based guideline update: Plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence-based guideline update: Plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology, 76(3):294-300. Mt Sinai J Med. 2011 Mar-Apr;78(2):161-75. doi: 10.1002/msj.20239.
31. Derwenskus J (2011). Current disease-modifying treatment of multiple sclerosis. Mount Sinai Med J, 78(2): 161-175.
32. Frohman T, Castro W, Shah A, Coutney A, Ortstadt J, Davis SL, Logan D, Abraham T, Abraham J, Remington T, Treadaway K, Graves D, Hart J, Stuve O, Lemack G, Greenberg B, Frohman EM (2011). Symptomatic treatment in multiple sclerosis. Ther Adv Neurol Disord, 4(2): 83-98.
Quote:MS is the most common immune disorder of the nervous system, and is a significant cause of disability in Western nations. Its cause remains obscure, but there has been an enormous advance in understanding of its pathophysiology and options for its treatment[1].
MS probably existed in ancient times, but clinical descriptions cannot be identified in ancient medical texts in the way that cases of epilepsy and dementia can. This may be because the complex and variable symptoms and signs of the disorder often resulted in its being diagnosed as something else, as has happened not infrequently in more recent times; it is also possible that the disease’s predilection for the temperate zones and northern climes made it less common in the African, Near Eastern and Southern European locations in which the earliest medical texts tended to be written [2]. One of the earliest clinical descriptions of what is probably MS was written around 1200 in Iceland about Halldora, a young woman with episodic visual loss and paralysis that remitted after prayers to the saints. A Dutch nun, Saint Lidwina of Schiedam, had intermittent paroxysmal pain, visual loss and leg weakness after falling while skating on a frozen canal at age 16; she died at 53 in 1433 after multiple such attacks, and became the patron saint of the chronically ill and of figure skaters. Reports of waxing and waning neurological signs and symptoms with a predilection for the visual pathways and the spinal cord came predominantly from northern Europe, and have led to the suggestion of a "Viking gene" that is responsible for the disease [3].
The clinical features and distinctive pathology of sclerose en plaque were delineated by Charcot in 1868. These features had been observed before, and also described in the writings of at least one patient, but Charcot’s contribution, as with so many other neurological diseases, was to synthesize them and to differentiate them from the signs and symptoms of other disorders and establish criteria for diagnosis of the disease. Robert Carswell in England and Jean Cruveilher in France had described the signs and symptoms of the disease and found scarring and atrophy of the spinal cord in some patients during the 1830s, but did not recognize this as a distinct disease. The Swiss anatomist Georg Rindfleisch showed in 1863 that inflammatory lesions of the spinal cord were often distributed around blood vessels, but did not draw conclusions from this. Charcot demonstrated the cardinal symptoms that became part of a diagnostic triad that bears his name – nystagmus, intention tremor and scanning or telegraphic speech – and also found that patients had slowing of cognition and enfeeblement of memory, which was not generally agreed upon as a manifestation of MS for another century[4].
Several literary descriptions of MS are in retrospect evident in the 19th century. Augustus Frederick d’Este, grandson of King George III, kept a detailed diary from 1822 until 1846 that described transient monocular visual loss, leg weakness, hand clumsiness, dizziness, numbness, unsteadiness requiring the use of a wheelchair, urinary incontinence and impotence consistent with MS. A later diarist, Bruce Frederick Cummings, wrote under the pen name of W.N.P. Barbellion an account of progressive neurologic deficit consistent with what is today called chronic progressive MS, that resulted in his death at age 31 in 1919 [5].
In the decades after Charcot’s description, salient features of MS were delineated by Walter Moxen in Britain and Edward Seguin in the United States: female predominance, generally non-inherited character and relapsing and remitting course. The causes of the disorder remained obscure, in part because the myelin sheath around nerve axons was not known until reported by Louis-Antoine Ranvier in 1878, and it was not until 1916 that James Dawson described the microscopic evidence of inflammation in myelin that is the hallmark of the MS plaque. Oligodendrocytes, the glial cells that produce myelin, were identified in 1928, and the role of the myelin sheath in rapid axonal transmission of nerve impulses (saltatory conduction) began to be understood at that time [6].
It was widely believed at that time that some unidentified toxin caused MS, and the occasional occurrence of MS-like "paralytic accidents" after rabies vaccinations, suggested that viral infection might play a role. An animal model (experimental allergic encephalomyelitis) was developed in the 1930s by Thomas Rivers and colleagues at Rockefeller University, and it was shown that immune cells and not viruses were responsible for the inflammatory attacks [7]. Seven decades of subsequent research have identified multiple components of the immune system that are responsible for autoimmune diseases: T-cell lymphocytes, antibodies, complement and chemokines [8].
In subsequent decades a number of other theories were proposed as to the cause and mechanisms of MS, such as chronic circulatory insufficiency due to arterial or venous disease or depletion of the gut biome causing susceptibility to infection or altered immune function. Various treatments including cerebral vasodilators, surgical procedures to improve venous drainage, radiation therapy and probiotics have been attempted. The major therapeutic advances, however, have involved modulation of the immune system with corticosteroids, plasmapheresis, interferon and its derivatives and the antineoplastic drug mitoxantrone. Recent attention has been focused on antibodies raised against attacking immune cells, neuroprotective agents and drugs that improve the ability of the damaged myelin sheath to conduct nerve impulses [9].
CLINICAL FEATURES
![[Image: 640px-Symptoms_of_multiple_sclerosis.svg.png]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/bf/Symptoms_of_multiple_sclerosis.svg/640px-Symptoms_of_multiple_sclerosis.svg.png)
"Symptoms of multiple sclerosis" by Mikael Häggström (Wikimedia)
- MS is classically defined by episodic neurologic deficit corresponding to lesions that are disseminated in time and space within the central nervous system.
- Almost any type of symptom can occur, but certain types or complexes of symptoms predominate and the attacks must last more than 24 hours.
- Another venerable clinical maxim is that MS causes symptoms without signs and signs without symptoms: patients will have neurological symptoms, sometimes quite intensely, that are not accompanied by abnormal findings on neurological examination, and in other cases neurological signs on examination will not be accompanied by patient complaints suggesting central nervous system dysfunction.
- The progression of physical and cognitive deficits may occur cumulatively over time without identifiable remissions or relapses [10].
Sensory loss or disturbance (paresthesias) are often the earliest complaint, as is twitching of the facial muscles (myokimia). Eye symptoms, particularly diplopia, occur in about a third of patients, pain in about half of patients during their clinical courses and constitutional symptoms, especially dizziness and fatigue, are reported by 70 per cent of patients. Heat intolerance, depression and less frequently euphoria and subjectice difficulties with memory or attention are commonly reported. Charcot described a symptomatic triad of ataxia due to cerebellar involvement, nystagmus and tremor which bears his name. Facial weakness, more often facial pain due to trigeminal neuralgia and spinal cord symptoms involving weakness, bowel and bladder disturbance, sexual dysfunction or transverse myelitis are also common [11].
Optic neuritis is the first clinical event in 20 per cent of patients and 40 per cent eventually experience it. Fatigue is reported by as many as 75 per cent of patients, and is the leading cause of disability in 50 to 60 per cent. Spasticity is nearly universal, and the increased muscle tone and greater difficulty of performing movements and activities contributes to fatigue. Forty to 70 per cent of MS patients have cognitive difficulties, chiefly affecting memory but also involving visual perception and executive function, that also adversely affect quality of life and contribute to disability. About half of patients have significant pain, which is mainly neuropathic in character and related to the demyelinating lesions, but can also over time arise from spasticity, abnormal posture or impaired balance. Urinary symptoms can involve failure of the bladder to store urine properly, resulting in frequency, urgency or incontinence, or failure to empty normally, causing urinary retention and infection. Constipation is the most common intestinal complaint, due either to neurogenic bowel dysfunction from spinal lesions or to bowel immobility. Aphasia and other language disturbances or seizures are rare, reported by less than 5 per cent of patients [12].
The physical and neurological examination is often abnormal in MS and may change from examination to examination, not always in conjunction with clinical symptoms. The most common abnormalities are localized weakness, generally with spasticity of the involved limbs; focal sensory impairment, particularly of posterior column modalities (vibration and proprioception); slowing and decreased coördination of limb movements and an ataxic wide-based gait ("spastic ataxia"). Optic neuritis commonly involves unilateral visual loss or impairment, sometimes painful, but is usually retrobulbar in location and may cause only pallor of the optic disc on fundoscopic examination ("the patient sees nothing and the doctor sees nothing"). Eye movements are frequently abnormal, most commonly inability to move one eye laterally due to abducens nerve palsy or bilateral internuclear ophthalmoplegia from involvement of the medial longitudinal fasciculus in the brain stem, resulting in inability to move the eye on the side of the lesion to the midline (adduction) along with nystagmus in the other (abducting) eye [13].
MS is clinically classified on the basis of relapses and remissions or their absence. Relapsing-remitting MS (RRMS) is the classical form, and accounts for about 85 per cent of cases. Studies with serial MRI imaging indicate that asymptomatic demyelinating episodes can occur in between clinical relapses. About half of patients with RRMS convert to a secondary progressive form (SPMS) after 10-15 years of disease; attacks no longer remit and there is progressive accumulation of disability. Primary progressive MS (PPMS) is progressive without remissions from the start, and represents about 10 per cent of cases; 5 per cent of patients have a progressive course with periodic superimposed relapses (PRMS). There are also milder variants: the clinically isolated syndrome (CIS) , also called "possible MS" involves a single MS-like attack and some patients have mild and infrequent attacks with complete resolution of symptoms and little or no accumulation of deficit over 15 to 20 years of disease (Benign MS) [14].
The severity and progression of MS are measured by disability scales, chiefly the Kurtzke Disability Status Scale, developed in 1955 and now considerably expanded (EDSS). Grades from 0 to 4 are determined by functional system (FS) scores for pyramidal (motor), cerebellar, brain stem, sensory, bowel and bladder and visual status as well as other aspects of neurologic function, particularly cognition. These yield scores ranging in increments of 0.5 from a score of 0 for normal neurologic examination and cerebral function to a score of 10 for death from MS. Another simple measure of disability is the Ambulation Index, which is the length of time required to walk 25 feet [15].
EPIDEMIOLOGY
MS is the most common autoimmune disorder involving the central nervous system, and affected 30 people per 100,000 with 2.5 new cases per 100,000 and 18,000 deaths in 2010. There are marked geographic and climate differences in prevalence, with 0.5 cases per 100,000 in Africa but 9 per 100,000 in the Americas and 80 per 100,000 in Europe generally, and 200 per 100,000 in northern European populations. Its onset is most commonly in the 20s and 30s, and at that age women predominate over men by about 2 to 1 and most cases are the classical RRMS. In cases with onset after age 50, incidence is about equal in women and men and PPMS is the most common form [16]. Individuals born in areas with high MS incidence who move to areas with low incidence before age 15 acquire the new region’s risk for MS, but if they migrate after age 15 they keep the MS risk of the area in which they were born. Many potential risk factors for MS have been scrutinized, generally with equivocal or results; there is an increased risk with smoking, and gout is less frequent among MS patients than would be expected while serum uric acid levels are lower than in individuals without MS [17].
GENETICS
Inheritance does not play a strong part in the acquisition of MS. The concordance rate between monozygotic twins is only 20 to 30 per cent, and the risk of developing the disorder is about 7 times greater for first-degree relatives like children and siblings than for the general population but the excess risk in families over a lifetime is only about 5 per cent. This is consistent with minimal genetic predisposition and greater effect from some environmental exposure, as is the occurrence of conjugal MS in husbands and wives who do not have any MS in their own biological families. Rather than gene mutations associated with the disease, it has been suggested that variants of genes (polymorphisms) may cause different degrees of gene expression in different people, and if genes regulating immune activity are involved, then some of those people may have exaggerated expression of a proinflammatory gene that results in untoward immune reactions and autoimmune disorders. A polymorphism may result in greater likelihood of developing MS or having a more severe or unremittent form of the disease if exposed to a trigger for the development of symptoms, while other polymorphisms might confer some degree of protection against the disorder.
Polymorphisms of the HLA genes located on chromosome 6 (MHC or major histocompatibility complex) have been linked to susceptibility to autoimmune diseases including MS, type 1 diabetes and systemic lupus erythematosus. Increased MS risk has been associated with MHC alleles DR15 and DQ6, while other HLA alleles have had an apparent protective effect against developing MS (HLA-C554 and HLA-DRB1*11. About half of the modest genetic predisposition to MS is apparently due to these HLA-MHC genes, while the rest is due to 12 or more genes in other locations, polymorphisms of which may increase the risk of developing MS [18].
CAUSES
If genetic factors predispose certain individuals to an excessive immune reaction and the development of autoimmune disorders, or in some cases genetic protection against immune overreaction is absent, there must still be some event or agent that triggers the immune process. Viral infections have been suggested to activate self-reactive T-cells that may react against myelin, and chronic virus infection with periodic reactivation could produce remissions and relapses of disease. Several viruses have been proposed as MS triggers, initially measles and canine distemper and most recently Epstein-Barr virus (EBV). MS patients have a high incidence of serum antibodies against EBV and EBV antigens are often expressed in MS plaques, but most people with EBV infection do not develop MS and many patients with MS do not have evidence of EBV infection [19].
The geographic predilection of MS has raised the question of environmental causes in northern latitudes. If there is an environmental factor involved, it must exert its effect early as moving to a new location after age 15 causes people to incur the MS risk of their new domicile thereafter; in addition, some ethnic groups in regions farther from the Equator that have high MS incidences do not themselves often develop MS, such as Canadian Inuits, American Eskimos, the Sami or Lapps of Finland and the Maori of New Zealand. There is also some evidence in recent studies that the north-south differential in MS incidence is decreasing [20]. Vitamin D levels have been proposed as a causative environmental factor and vitamin D has a role in regulating immune response by decreasing the production of proinflammatory cytokines and increasing the production of anti-inflammatory cytokines. High circulating levels of vitamin D appear to be associated with a reduced risk of MS, and while vitamin D might be deficient in some northern areas that have less sunlight and higher MS incidence, studies suggest that Norway, where the traditional diet is very high in vitamin D, has a lower incidence of MS than its Scandinavian neighbors [21].
Hepatitis vaccination and venous insufficiency are recent suggestions for possible causative factors in MS that have not yet gained wide acceptance. Anecdotal reports that MS was more frequent after hepatitis vaccination prompted investigation by the Centers for Disease Control and Prevention (CDC), but an increased incidence of the disease among hepatitis-vaccinated individuals was not confirmed [22]. Several studies have suggested that venous stenosis and insufficient cerebral venous drainage can lead to venous stasis, iron deposition in the brain and the triggering of an immune reaction causing MS. Internal jugular vein-azygos vein angioplasty was reported to alleviate MS symptoms, but this study has not yet been replicated [23].
DIAGNOSIS
MS has historically been diagnosed by history and neurological findings. Current criteria specify that there have been two or more objectively evident attacks at different times, two separate episodes with clinical evidence from one and MRI abnormality ascribable to the other, one objectively-verifiable clinical attack with MRI evidence of two or more others in the past, a single episode of neurological deficit (clinically isolated syndrome) followed by a later episode or MRI abnormality or insidious progression of neurological deficit over a year accompanied by one brain lesion on MRI, two spinal cord lesions or abnormal cerebrospinal fluid studies [14].
MRI imaging is highly sensitive to the lesions of MS, being positive for 90 to 95 per cent of brain lesions and 75 per cent of spinal cord lesions, more in older than in younger patients. T2-weighted images show "bright objects" caused by edema associated with lesions, while T1-weighted images detect "black holes" from axonal death and resultant cerebral atrophy. Functional MRI and Magnetic Resonance spectroscopy may detect changes in patients with slight or absent changes on structural brain MRI or minimal clinical findings despite substantial MRI lesions. The current focus in MS neuroimaging is on correlation with therapeutic response [24].
Lumbar puncture was once routinely done and remains the only objective test for central nervous system inflammation; it is often helpful in patients with possible MS. Oligoclonal bands are present in 90 to 95 per cent of patients, and 70 to 90 per cent of those with MS will have intrathecal production of IgG [25]. Standard EEG is variably and nonspecifically abnormal in MS, but computer-assisted quantitative EEG shows differences between MS with benign prognosis and relapsing-remitting forms [26]. Evoked potentials to visual, auditory and somatosensory stimulation extracted from the EEG by signal averaging have been largely superceded by MRI, but may show separate lesions and predict MS course and effect of therapy [27].
Cerebral angiography is rarely used to differentiate MS and cerebral vasculitis. Ultrasound examination has been suggested for measurement of ventricular size in the evaluation of cognitive dysfunction in MS. Laboratory studies are appropriate to exclude differential possibilities such as Lyme disease, syphilis, thyroid disease, vitamin B12 deficiency and Wilson’s disease; Devic’s disease or neuromyelitis optica, which can more closely resemble MS, is associated with antibodies to the nerve membrane water channel aquaporin 4 [28].
TREATMENT
For about a century there was no effective treatment for MS. In the last 25 years there have been substantial advances in its management, due in large part to biotechnology advances in the areas of neuroimaging, drug development, genetics and immunology. MS therapy now involves disease-modifying or immunomodulatory treatments to reduce the frequency and severity of relapses and slow progression of the disease, and symptomatic management of the effects of demyelination or neurological deficit. With one exception, disease-modifying drugs are approved only for the relapsing form of MS. Symptomatic management focuses on spasticity, bowel and bladder disturbances, effects of paralysis and immobility, alleviation of pain and amelioration if possible of cognitive and behavioral problems. There is evidence that prompt treatment of clinically isolated symptoms reduces the conversion of possible to probable or definite MS by about 40 per cent [29].
Acute relapses have historically been treated with corticosteroids, first ACTH and more recently methylprednisolone. This alleviates acute symptoms but may not change overall disease progression, and it is not clear that it works for optic neuritis. Plasmapheresis is considered probably effective as second-line treatment when steroids do not work or cannot be used [30].
Ten immunomodulatory drugs are now approved for relapsing MS in the United States. Three (fingolimod, teriflunomide and dimethyl fumarate) are taken orally, interferon-beta-1a is administered intramuscularly, pegylated interferon-beta-1a (mixed with PEG or polyethylene glycol for longer duration of action), interferon-beta-1b and glatirimer acetate are given subcutaneously and natalizumab and mitoxantrone require intravenous infusion [31].
Interferon-beta-1b (Betaseron) was the first agent to be approved in 1993, and decreased the frequency of relapses by about a third, significantly reduced the acquisition of MS lesions and brought about a 10 per cent reduction in the incidence of disease progression. Side effects include asthenia, fatigue, depression, increased muscle tone, skin reactions at the injection site, leukopenia and increase liver enzymes, myasthenia and most commonly, flu-like symptoms.
Intramuscular interferon-beta-1a (Avonex) decreased the annual exacerbation rate by 29 per cent, reduced disease progression from 35 per cent in the placebo group to 21 per cent in the treatment group and significantly decreased the number and volume of MRI enhancing lesions. The subcutaneous form (Rebif) reduced relapses by 27 (low dose) and 33 (high dose) percent, and was shown in other studies to reduce cumulative disability and the burden of MRI lesions with the higher dose. Flu-like symptoms were less than with interferon-beta-1b but injection site reactions, white blood cell abnormalities and liver disorders were more common. The subcutaneous preparation had fewer flu-like reactions, but skin reactions and abnormalities of liver function and white blood cells were more common. Both forms of interferon-beta-1a were associated with neutralizing antibodies which lessened drug effect, and this was more common with the subcutaneous form. Interferon-beta-1a admixed with PolyEthylene Glycol (PEGylated and called Plegridy) can be self-administered subcutaneously every 2 weeks, and reduced the relapse rate by 36 per cent, decreased disability progression by 38 per cent and lessened the appearance of new MRI lesions by 67 to 85 per cent as compared to placebo. Interferon-beta-1may be problematic in patients with uncontrolled depression, and consideration of glatirimer is recommended instead.
Glatirimer acetate (Copaxone) is a synthetic polypeptide that produced a 29 per cent reduction in relapse rate over 2 years and may have slightly reduced disability; it was therefore approved by the Food and Drug Administration for relapse prevention but not for slowing disability progression. A higher dose approved in 2014 may be more effective, and can be administered subcutaneously 3 times a week rather than once daily.
Natalizumab (Tysabri) is a monoclonal antibody, hence the "mab" in its name, that binds to the adhesion molecule alpha-4 integrin and prevents it from adhering to receptors and presumably from initiating an immune response. It is raised in mice and can therefore be immunogenic itself, so it is altered and "humanized" for monthly intravenous infusion. Natalizumab reduced relapses by 68 per cent and disease progression by 42 per cent over 2 years, but was also associated with fatal cases of the opportunistic viral infection progressive multifocal leukoencephalopathy and was therefore withdrawn in 2005. It was reintroduced in 2006 for patients with very active relapsing disease or no response to interferon or glatirimer. It may also work for primary and secondary progressive MS, but is available only through a restricted distribution system.
Fingolimod (Gilenya) is derived from miryocin, produced by a fungus related to several used in Chinese and Tibetan medicine. Fingolimod is an analogue of the cell membrane lipid sphingosine, and through sphingosine-1-phosphate receptors causes lymphocytes to remain in lymph nodes so they cannot initiate an immune attack; it may also stimulate the repair and replacement of glial cells, perhaps including the ones which synthesize myelin. It was the first MS drug that can be taken orally, and in daily administration significantly reduced relapses and delayed the acquisition of disability. Reduced lymphocyte count and resultant infection, macular edema, skin cancer and bradycardia and hypotension have occurred, the latter sufficiently severe that the first doses should be taken under medical observation and an ECG obtained before and after. The drug is now contraindicated in patients with cardiovascular disease.
Teriflunomide (Aubagio) inhibits the rapid division of cells, and may therefore retard the activation of T-cells to start an immune response. It produced a 31 per cent reduction in relapse rate and minimally decreased the progression of disability, and significantly reduced the development of definite MS in patients with clinically isolated syndromes. Decreased white blood cell count and increased blood pressure may occur along with flu-like symptoms and other nonspecific effects; liver toxicity, teratogenesis, peripheral neuropathy, acute renal failure, immunosuppression with infection and particularly activation of latent tuberculosis, skin hypersensitivity reactions, interstitial lung disease, hyperkalemia and hypophosphatemia. It is contraindicated in patients with liver disease, women of childbearing age who are not sterilized or using contraceptives and those taking its parent compound leflunomide (Arava) for arthritis.
Dimethyl fumarate (Tecfidera) was approved for oral treatment of relapsing MS because it decreased the proportion of patients who relapsed by half, decreased the annual rate of relapse by 53 per cent and caused a 38 per cent reduction in progression of disability over 2 years as compared to placebo. The appearance of MRI lesions was also significantly reduced compared to placebo. The drug activates the nuclear factor (erythroid-derived 2)-like 2 or Nrf2 pathway that regulates the synthesis of antioxidant proteins that protect against cell injury and inflammation, and may therefore have a neuroprotective effect. It is also an agonist of the nicotinic acid receptor, which may be helpful for oxidative cell stress and could account for the side effects of flushing, gastrointestinal upset, diarrhea and nausea. White blood cell count and liver function tests need to be monitored also.
The most recent addition to the disease-modifying armamentarium is alemtuzumab (Lemtrada), developed for chemotherapy of leukemia and lymphoma and also used as an immunosuppressant in preparation for kidney, islet cell and bone marrow transplantation. It is a recombinant monoclonal antibody against the CD52 lymphocyte antigen, and cells to which it binds are targeted for destruction. As a result, the risk of severe autoimmune reactions is significant, but the drug was significantly more effective than interferon-beta-1a or 1b in patients new to treatment or relapsing during treatment. In a later study, a quarter of the patients treated with alemtuzumab became free of disease activity (no relapses, no accumulation of disability, no new MRI lesions) while none of the interferon-treated patients did.
Mitoxantrone (Novantrone) is approved for secondary progressive MS, relapsing MS that is worsening and for progressive relapsing disease. It is an immunosuppressive agent used for lymphocytic leukemia, lymphoma and breast cancer, and has been given "black box" warnings by the FDA on account of cardiac toxicity and risk of developing acute myelogenous leukemia. Some of the same adverse effects, along with hemorrhagic cystitis, are associated with cyclophosphamide (Cytoxan) therapy, but this immunosuppressive agent is effective in inducing remission of aggressive relapsing or secondary progressive MS, followed by maintenance therapy with glatirimer. No therapy is presently approved for primary progressive MS.
Pharmacological treatment, physical and occupational therapy, speech therapy and cognitive-behavioral psychotherapy have been shown to be effective for the management of symptoms and disability. The areas in which symptomatic therapy is appropriate include visual impairment from optic neuritis, fatigue, spaticity, bowel and bladder dysfunction, cognitive decline, depression and less frequently agitation, paroxysmal disturbances and particularly pain, tremor, heat intolerance and sexual dysfunction32. If not treated in one way or another, about a third of patients will have permanent physical disability after 20 to 25 years of disease. Five to 10 per cent will manifest apparently benign disease, with little or no disability after a long course, but these patients may show cognitive deterioration over time. Patients with primary progressive disease or spinal cord lesions from MS will have the worst prognosis, particularly males. Life expectancy is in general only modestly shortened, and death is caused by secondary infectious, pulmonary or renal complications in about two-thirds of patients. Although MS remains a significant long-term treatment and management problem, the accelerating pace of diagnostic and therapeutic development, due in large part to advances in genetic and drug development technologies, has improved its outlook over the past 25 years.
REFERENCES
1. Compston A, Coles A (2008). Multiple sclerosis. Lancet, 372(9648): 1502-1517.
2. Maeder R (1979). Does the history of multiple sclerosis go back as far as the 14th century? Acta Neurol Scand, 60 (3): 189–92.
3. Holmøy T (2006). A Norse contribution to the history of neurological diseases. Eur. Neurol. 55 (1): 57–8.
4. Murray TJ (2005). Multiple Sclerosis: The History of a Disease. New York, Demos Press.
5. Orrell RW (2005). Multiple Sclerosis: The history of a disease (review). J Roy Soc Med, 98(6): 289.
6. Piccolino M (2003). Nerves, alcohol and drugs, the Adrian-Kato controversy on nervous conduction: deep insights from a "wrong" experiment? Brain Res Rev, 43(3): 257-265.
7. Van Epps HL (2005). Thomas Rivers and the EAE model. J Exp Med, 202(1): 4.
8. Steinman L (2003). Optic neuritis, a new variant of experimental encephalomyelitis, a durable model for all seasons, now in its seventieth year. J Exp Med, 197(9): 1065-1071.
9. Sontheimer H (2015). Multiple sclerosis. In, Diseases of the Nervous System. Waltham MA; Academic Press. New diagnostic criteria for multiple sclerosis: guidelines for research protocols.
10. Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtelotte WW (1983). New diagnostic criteria for multiple sclerosis: guideline for research protocols. Ann Neurol, 13(3): 227-231.Ann Neurol. 1983; 13(3):227-31.
11. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sanberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001). Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol, 50(1):121-127.
12. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sanberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005). Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol, 58(6): 840-846.
13. National Clinical Guideline Center (2014). Management of Multiple Sclerosis in Primary and Secondary Care (NICE Clinical Guidelines, No. 186). London: National Institute for Health and Care Excellence (UK).
14. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA. Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L. Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol, 69(2):292-302.
15. Kurtzke JF (1983). Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology, 33(11):1444-52.
16. Milo R, Kahana E (2010). Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun Rev, 9(5): A387-A394.
17. Ascherio A, Munger KL (2007). Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol, 61(6): 504-513
18. Baranzini SE (2011). Revealing the genetic basis of multiple sclerosis: are we there yet? Curr Opin Genet Devel, 21(3): 317-324.
19. Ascherio A, Munger KL (2007). Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol, 61(4): 288-299.
20. Salvetti M, Giovannoni G, Aloisi F (2009). Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol, 22(3):201-6.
21. Kampman MT, Brustad M (2008). Vitamin D: a candidate for the environmental effect in multiple sclerosis - observations from Norway. Neuroepidemiology, 30(3):140-6.
22. Centers for Disease Control and Prevention (2010). FAQs about Hepatitis B Vaccine (Hep B) and Multiple Sclerosis. Bethesda, MD. US Department of Health and Human Services.
23. Lapaucis A, Lillie E, Dueck A, Straus S, Perrier L, Burton JM, Aviv R, Thorpe K, Feasby T, Spears J (2011). Association between chronic cerebrospinal venous insufficiency and multiple sclerosis: a meta-analysis. CMAJ, 183(16):E1203-12.
24. Sicotte NL (2011). Neuroimaging in multiple sclerosis: neurotherapeutic implications. Neurotherapeutics, 8(11): 54-62. therapeutics. 2011 Jan;8(1):54-62.
25. Dobson R, Ramagopalan S, Davis A, Giovannoni G (2013). Oligoclonal bands in multiple sclerosis and clinically isolated syndromes. Cerebrospinal Fluid Oligoclonal Bands in Multiple Sclerosis and Clinically Isolated Syndromes. A meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiat, 84(8): 909-914.
26. Vazquez-Marrufo M, Gonzalea-Rosa JJ, Vaquero E, Duque P, Borges M, Gomez C, Izquierdo G (2008). Quantitative electroencephalography reveals different physiological profiles between benign and remitting-relapsing multiple sclerosis patients. Quantitative electroencephalography reveals different physiological profiles between benign and remitting-relapsing multiple sclerosis patients. BMC Neurol, 8:44.
27. Margaritella N, Mendozzi L, Garegnani M, Nemni R, Colicino E, Gilardi E, Pugnetti L (2012). Exploring the predictive value of the evoked potentials score in MS within an appropriate patient population. A hint for an early identification of benign MS? BMC Neurology, 12: 80.
28. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005). IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med, 202(4): 473-477.
29. Freedman MS (2013). Teriflunomide in relapsing multiple sclerosis: therapeutic utility. Ther Adv Chron Dis, 4(5): 192-205.
30. Cortese I, Chaudhry V, So YT, Cornblath DR, Rae-Grant A (2011). Evidence-based guideline update: Plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence-based guideline update: Plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology, 76(3):294-300. Mt Sinai J Med. 2011 Mar-Apr;78(2):161-75. doi: 10.1002/msj.20239.
31. Derwenskus J (2011). Current disease-modifying treatment of multiple sclerosis. Mount Sinai Med J, 78(2): 161-175.
32. Frohman T, Castro W, Shah A, Coutney A, Ortstadt J, Davis SL, Logan D, Abraham T, Abraham J, Remington T, Treadaway K, Graves D, Hart J, Stuve O, Lemack G, Greenberg B, Frohman EM (2011). Symptomatic treatment in multiple sclerosis. Ther Adv Neurol Disord, 4(2): 83-98.
by SunilNagpal at 05-21-2015, 04:43 AM
4 comments
As per recent media reports, recent quality audits conducted at Maggi production units of Nestle in some parts of Uttar Pradesh, India have revealed some startling facts about Maggi. MSG (a potent allergen to many people) and Lead (a highly potent carcinogen)have been found to be in high concentrations (well above permissible limits). Reports have indicated the presence of lead at as high as 17ppm whereas the permissible concentration is not more than 0.01 ppm!
FSDA (Food and Drug Safety Administration) of Uttar Pradesh has reportedly written to Food Safety and Standard Authority of India (FSSAI) to take immediate action and ban the production.
Let's wait and watch the fate of '2 minutes Maggi Noodles' in India.
Till then stay safe, eat safe and live safe.
NOTE:
These are not my personal views/stories, I read it in TOI. Entire Indian Media is abuzz with this development! Do share your views and information about this concerning development.
FSDA (Food and Drug Safety Administration) of Uttar Pradesh has reportedly written to Food Safety and Standard Authority of India (FSSAI) to take immediate action and ban the production.
Let's wait and watch the fate of '2 minutes Maggi Noodles' in India.
Till then stay safe, eat safe and live safe.
NOTE:
These are not my personal views/stories, I read it in TOI. Entire Indian Media is abuzz with this development! Do share your views and information about this concerning development.
by iritechinc at 05-19-2015, 01:55 PM
3 comments
According to The Global Government Biometric Systems Market 2015-2025 report of MarketResearchReports published last month, due to the increasing concerns over internal and external security and the increasing use in government and private sectors for employee identification and attendance, the global biometrics market is forecasted to rise in the demand and revenue in the next few years. In terms of geography, the biometrics market is expected to be continuously dominated by North America, followed by Asia Pacific and Europe. However, the growth rate of biometric market in the Asia-Pacific region is expected to overtake North America by 2015.
There are many factors that boost the growth of Asia-Pacific biometric market.
Firstly, governments in the Asia-Pacific region have introduced several projects in terms of national identities and border administration. In 2012, China introduced the new China Resident Identity Card Law. Under this new rule, Chinese citizens are required to have their fingerprints scanned and recorded as part of any subsequent administrative transaction involving their resident ID cards. Hence, the expectation of high level of certification will be required for the biometric hardware being leveraged in these enrollment operations. Moreover, initiatives such as e-KTP electronic ID initiative in Indonesia and the UIDAI project in India using face, fingerprints and iris biometrics for individual identification are also expected to provide new opportunities for the biometrics technology market in Asia-Pacific.
Secondly, the Asia-Pacific region is demonstrated to increase in security and IT spending. According to the Frost & Sullivan Aerospace & Defense and Asia-Pacific consultant, Amartya De, terrorist threats combined with increased air traffic are driving increased aviation security spending in this region. Amartya De also states that overall homeland security spending across Asia-Pacific is asserted to be US$30 billion in 2014. Specifically, airport security market is expected to grow to US$9.23 billion by the end of 2015. Airport security spending in Australia is reported to reach US$640.2 million by the end of 2015, and by which time spending in Singapore will reach US$298 million. This security spending segment is indicated to dominate the security expenditure in the Asia Pacific region, which forces the rapid growth of the biometric-based technologies in this area.
Ultimately, the growth of the Asia-Pacific electronic access control (EAC) systems market is also the key factor fueling the rise of biometric in Asia-Pacific. Due to the concerns over the enhancing security and reducing fraud and identity theft across government and commercial setting, the demand of electronic access control systems in Asia-Pacific continuously increases. Based on “Global Market Study on Electronic Access Control (EAC) Systems: Biometric Systems to Witness Highest Growth by 2019” report, The Asia-Pacific EAC systems market is predicted to record the highest CAGR growth of 16.7% to reach USD 9.6 billion in 2019. The rapid growth rate of EAC systems contributes to the prevailing future of Asia-Pacific biometric market versus North America.
According to the Strategic Defense Intelligence, it is estimated that the biometric market in the Asia-Pacific region to value US$1.1 billion in 2015 and reach US$3.3 billion by 2025, growing at a CAGR of 11.3% during this period. China and India are the biggest contributors to this growth, occupying more than half of the regional market share. Besides, Japan and South Korea are also the prime revenue generating countries in Asia-Pacific biometrics market. Other countries such as Australia, Philippines, Sri Lanka, Bangladesh, Thailand and Malaysia are expected to play vital role in the growth of overall Asia-Pacific biometric market in the forecast period. For example, Sri Lanka and Philippines have been initiating biometrics-based passports since 2014 and 2015, respectively, and encouraging the demand for biometrics devices for identity solutions in these countries.
There are many factors that boost the growth of Asia-Pacific biometric market.
Firstly, governments in the Asia-Pacific region have introduced several projects in terms of national identities and border administration. In 2012, China introduced the new China Resident Identity Card Law. Under this new rule, Chinese citizens are required to have their fingerprints scanned and recorded as part of any subsequent administrative transaction involving their resident ID cards. Hence, the expectation of high level of certification will be required for the biometric hardware being leveraged in these enrollment operations. Moreover, initiatives such as e-KTP electronic ID initiative in Indonesia and the UIDAI project in India using face, fingerprints and iris biometrics for individual identification are also expected to provide new opportunities for the biometrics technology market in Asia-Pacific.
Secondly, the Asia-Pacific region is demonstrated to increase in security and IT spending. According to the Frost & Sullivan Aerospace & Defense and Asia-Pacific consultant, Amartya De, terrorist threats combined with increased air traffic are driving increased aviation security spending in this region. Amartya De also states that overall homeland security spending across Asia-Pacific is asserted to be US$30 billion in 2014. Specifically, airport security market is expected to grow to US$9.23 billion by the end of 2015. Airport security spending in Australia is reported to reach US$640.2 million by the end of 2015, and by which time spending in Singapore will reach US$298 million. This security spending segment is indicated to dominate the security expenditure in the Asia Pacific region, which forces the rapid growth of the biometric-based technologies in this area.
Ultimately, the growth of the Asia-Pacific electronic access control (EAC) systems market is also the key factor fueling the rise of biometric in Asia-Pacific. Due to the concerns over the enhancing security and reducing fraud and identity theft across government and commercial setting, the demand of electronic access control systems in Asia-Pacific continuously increases. Based on “Global Market Study on Electronic Access Control (EAC) Systems: Biometric Systems to Witness Highest Growth by 2019” report, The Asia-Pacific EAC systems market is predicted to record the highest CAGR growth of 16.7% to reach USD 9.6 billion in 2019. The rapid growth rate of EAC systems contributes to the prevailing future of Asia-Pacific biometric market versus North America.
According to the Strategic Defense Intelligence, it is estimated that the biometric market in the Asia-Pacific region to value US$1.1 billion in 2015 and reach US$3.3 billion by 2025, growing at a CAGR of 11.3% during this period. China and India are the biggest contributors to this growth, occupying more than half of the regional market share. Besides, Japan and South Korea are also the prime revenue generating countries in Asia-Pacific biometrics market. Other countries such as Australia, Philippines, Sri Lanka, Bangladesh, Thailand and Malaysia are expected to play vital role in the growth of overall Asia-Pacific biometric market in the forecast period. For example, Sri Lanka and Philippines have been initiating biometrics-based passports since 2014 and 2015, respectively, and encouraging the demand for biometrics devices for identity solutions in these countries.
by StutiB at 05-18-2015, 01:09 AM
3 comments
Hello, can anyone share their written test/interview experience for admission to the M.Tech Biotechnology program @IIT Guwahati? What are the main topics one should focus on for the written test and is it all multiple choice?
by Rivitra d/o Vintisen at 05-12-2015, 07:16 PM
0 comments
I would like to study about plants.therefore i'm planing to do degree in agriculture. but some people do suggest me to do biotechnology. i would like to learn agriculture in India or Taiwan. So how do i pursue in order to reach that??
by Miles E. Drake at 05-12-2015, 01:38 PM
0 comments
Parkinson's disease (also called Paralysis agitans or shaking palsy) is a progressive neurological disorder that mainly affects the motor system but can cause cognitive disturbance and autonomic nervous system dysfunction as well. It is related to several dopamine neurotransmission systems in the brain and can probably arise from several different causes. Although incurable it is treatable with increasing effectiveness and tolerability by medication, neurosurgical procedures, brain stimulation and regenerative therapy.
INTRODUCTION:
There are many ancient descriptions of Parkinson’s disease, including passages in the Ebers medical papyrus from ancient Egypt, Ayurvedic texts from India, the Bible and the writings of Galen. There were no clear references to parkinsonism in medieval and Renaissance texts, but discussions of parkinsonian symptoms were published by the Dutch anatomist Sylvius and the English surgeon John Hunter during the 17th and 18th centuries.
Parkinson’s essay prompted communications from a number of British, French and German clinicians, and between 1868 and 1881 Jean-Martin Charcot elucidated the clinical features of several types of paralysis agitans and proposed that the syndrome be named after Parkinson [2].
CLINICAL FEATURES
Parkinson’s disease has primarily motor symptoms, but since Parkinson’s initial description, non-motor manifestations have been increasingly recognized and may in fact precede motor symptoms.
The motor hallmarks of the disorder are:
a) Bradykinesia or hypokinesia (slowness or reduction of movement)
b) Rigidity (increased muscle tone and resistance to movement due to continuous muscle contraction)
c) Tremor (involuntary rhythmic movement of the limbs when at rest)
d) An abnormal and unstable posture
Non-motor symptoms are numerous and include:
a) Dementia and behavioral or emotional disturbances
b) Autonomic nervous system dysfunction
c) Abnormalities of vision and eye movements [7].
![[Image: parkpic1335108259020.png]](http://classconnection.s3.amazonaws.com/762/flashcards/918762/png/parkpic1335108259020.png)
MOTOR SYMPTOMS
Effect on Movements
Muscle Rigidity in Parkinson's Disease
Tremors in Parkinson's Disease
Postural Abnormalities in Parkinson's Disease
NON-MOTOR SYMPTOMS
EPIDEMIOLOGY
GENETICS
About 15 per cent of parkinsonian patients have a first-degree relative with Parkinson’s disease, and heredity because of an identifiable genetic mutation has been shown in 5 per cent.
CAUSES
![[Image: parkinsons-disease.jpg]](http://callmyfamilydoctor.com/blog/wp-content/uploads/2015/01/parkinsons-disease.jpg)
Source: callmyfamilydoctor.com
DIAGNOSIS
TREATMENT
Great advances have been made in the past 50 years in treatment of Parkinson’s disease.
Recent studies of immunotherapy and neuroprotection have also attracted attention.
Medical approaches have focused on dopamine replacement or augmentation. L-DOPA (levodopa) is converted to dopamine by the enzyme DOPA decarboxylase in dopaminergic neurons, and has been used since in 1970s to replace depleted dopamine and alleviate motor symptoms. Since only about 5 per cent of L-DOPA crosses the blood-brain barrier and the remainder causes peripheral symptoms of dopamine excess such as nausea, peripheral DOPA decarboxylase inhibitors such as carbidopa and benserazide have been given along with levodopa or added to combination preparations such as Sinemet, the combination of carbidopa and levodopa which was named from the Latin for “without vomiting”. With increasing duration of treatment, patients may become less responsive to levodopa or have disabling fluctuations between rigidity and hyperkinesis, the “on-off” phenomenon. These were first addressed by “drug holidays”, periods in which levodopa was withdrawn, but opinion has turned against this because dyskinesias may become worse and life-threatening neuroleptic malignant syndrome may occur when the dopamine replacement is restarted.
Adjunctive drugs that inhibit the enzyme catechol-o-methyltransferase (COMT), which breaks down levodopa, may prolong and stabilize the dopaminergic effect. The first such drug, tolcapone, proved to have potentially fatal liver toxicity and has been withdrawn in most countries and is very rarely used in the others. Entacapone does not have the same toxicity, and is used by itself or in a combination preparation with levodopa and carbidopa. Another way to augment the effect of levodopa is to slow the metabolism of the dopamine it is made into by inhibiting monoamine oxidase B (MAO-B). This can be done with the vitamin E derivatives seligiline and rasagaline.
Direct stimulation of the dopamine receptor may be an alternative to levodopa, and have been shown to delay the onset of motor complications like dyskinesias and “on-off”. Bromocriptine, pergolide, pramipexole, ropinirole and cabergoline, along with piribedil outside the United States, can be taken orally, while apomorphine is given by injection and two receptor agonists (lisuride and rotigotine) can be administered through skin patches. These are increasingly used in the attempt to avoid levodopa early in the disease and to lessen motor complications later.
Surgical treatments began with lesions of the corticospinal tract to control tremor. With the introduction of the anticholinergic drugs such procedures declined, and lesions were occasionally made in selected motor areas in refractory patients, chiefly to diminish disabling dyskinesias. Pallidotomy, the cauterization of a small part of the globus pallidus, which is adjacent to the substantia nigra and through which most of its motor nerve output passes, has been the most common lesional operation for the combination or rigidity, tremor and dyskinesia. Lesioning of the thalamus (thalamotomy) with electrical cauterization or the application of a supercooled probe has also been carried out, particular for severe tremor, and has mainly focused on the subthalamic nucleus on the side opposite to the tremor. The operations are usually done under local anesthesia, as the brain itself is insensitive to pain, and the effect of the lesion on motor symptoms can be immediately assessed. The complications are those of craniotomy, chiefly bleeding and infection.
Deep Brain Stimulation grew out of lesioning surgeries, and was approved in the United States for tremor in 1997 and Parkinson’s disease in 2002, as well as for dystonia, obsessive-compulsive disorder, refractory depression and chronic pain. An implantable pulse generator in titanium housing is placed below the clavicle or in the abdomen, and is connected to a polyurethane-insulated coiled wire with four electrodes made of platinum and iridium, that are placed in one or two target nuclei. The electrodes are placed in the globus pallidus or subthalamic nucleus. The implantation is often done under general anesthesia with the device and the target nuclei visualized by intraoperative MRI, after which the effects of stimulation on tremor and dyskinesia can be calibrated when the patient is awake. The potential risks are again infection, estimated at 3 to 5 per cent, and hemorrhage in 1 or 2 per cent. There has also been interest in noninvasive brain stimulation through the skull with the transcortical magnetic device approved for refractory depression and migraine; some improvement in levodopa-related dyskinesia has been reported [23].
![[Image: 161px-Parkinson_surgery.jpg]](http://upload.wikimedia.org/wikipedia/commons/thumb/9/91/Parkinson_surgery.jpg/161px-Parkinson_surgery.jpg)
Electrode insertion during deep brain stimulation (Source: wikimedia)
New developments include promising attempts at treatment derived from the evidence that the accumulation of proteins normally disposed of may be harmful or fatal to the neurons of the substantia nigra by inciting an immune response, by triggering the processes of cell death that are programmed into all tissues or by the lack or loss of trophic factors or substances that protect neurons against this process. After promising studies in which monoclonal antibodies were targeted with beneficial effect against beta-amyloid, the protein whose inappropriate accumulation may be responsible for Alzheimer’s disease, studies are underway of passive and active immunization against the alpha-synuclein which accumulates in neurons in parkinsonism with apparent fatal effect. Active immunization involves injecting a small fraction of synthetic alpha-synuclein to induce the formation of antibodies, while passive immunization involves the preparation of monoclonal antibodies against alpha-synuclein which are then infused. The third approach is similar to recent advances in the treatment of amyotrophic lateral sclerosis, and involves transforming pluripotent adult stem cells into cells capable of secreting nerve growth factors known to be protective against cell death, then reinfusing these stem cells into the patient to offer neuroprotection to the imperiled dopaminergic neurons. These therapeutic options are now approaching or beginning phase 2 trials in hopes of demonstrating efficacy against Parkinson’s disease [20].
REFERENCES
1. Wilkins RH, Brody IA (1997). Neurological Classics. New York, Thieme. Pp. 87-92.
2. Lees AJ (2007). Unresolved issues related to the shaking palsy on the celebration of James Parkinson’s 250th birthday. Movement Disord, 22(suppl 17): S327-334.
3. Fahn S (2008). The history of dopamine and levodopa in the treatment of Parkinson’s disease. Movement Disord, 23(suppl 3): S497-508.
4. Lanska DJ (2010). The history of movement disorders. Handbook Clin Neurol, 95: 501-546.
5. Coffey RJ (2009). Deep brain stimulation devices: a brief technical history and review. Artif Organs, 33(3): 208-220.
6. Pollitis M, Lindvall O (2012). Clinical application of stem cell therapy in Parkinson’s disease. BMC Medicine, 10: 1.
7. Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA (2009). Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol, 8(12): 1128-1139.
8. Jankovic J (2008). Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiat, 79(4): 368-376.
9. Fung VS, Thompson PD (2007). Rigidity and spasticity. In, Tolosa E, Jankovic J (eds). Parkinson’s Disease and Movement Disorders. Hagerstown MD, Lippincott Williams & Wilkins, pp. 504-513.
10. Cooper G, Eichhorn G, Rodnitzky RL (2008). Parkinson’s disease. In, Conn PM (ed). Neuroscience in Medicine. Totowa NJ, Humana Press, pp. 508-512.
11. Yao SC, Hart AD, Terzella MJ (2013). An evidence-based osteopathic approach to Parkinson’s disease. Osteopath Fam Phys, 5(3): 96-101.
12. Murray ED, Buttner EA, Price BH (2012). Depression and psychosis in neurological practice. In, Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds). Bradley’s Neurology in Clinical Practice, ed. 6. Philadelphia, Elsevier-Saunders, pp. 102-103.
13. Ceravolo R, Frosini D, Rossi C, Bonuccelli U (2009). Impulse control disorders in Parkinson’s disease: definition, epidemiology, risk factors, neurobiology and management. Parkinson Relat Disord, 15(suppl 4): S111-115.
14. Friedman JH (2010). Parkinson’s Disease psychosis 2010: A review article. Parkinson Relat Disord, 16(9): 553-560.
15. Caballol N, Martí MJ, Tolosa E (2008). Cognitive dysfunction and dementia in Parkinson disease. Movement Disord, 22 (suppl 17): S358-366.
16. Poewe W (2006). The natural history of Parkinson’s Disease. J Neurol, 253(7 suppl): vii2-vii6.
17. Wright-Willis A, Evanoff BA, Lian M (2010). Geographic and ethnic variation in Parkinson disease: a population-based study of U.S. Medicare beneficiaries. Neuroepidemiol, 34: 143-151.
18. Shulman JM, De Jager PL, Feany MB (2011). Parkinson’s Disease: genetics and pathogenesis. Ann Rev Pathol, 6: 193-222.
19. Sontheimer H (2015). Diseases of the Nervous System. Waltham, MA, Elsevier. Pp. 134-166.
20. Stetka BS, Facheris M (2015). Prions, the pipeline and the latest in Parkinson’s disease. Medscape Neurology, May 8, 2015.
21. Brooks DJ (2010). Imaging approaches to Parkinson’s Disease. J Nucl Med, 51(4): 596-609.
22. Lingor P, Liman J, Kallenberg K, Sahlmann C-O, Bähr M (2011). Diagnosis and differential diagnosis of Parkinson’s Disease. In, Rana AQ (ed). Diagnosis and Treatment of Parkinson’s Disease. Rijeka, Croatia, InTech,com, pp. 1-20.
23. Koch G (2010). rTMS effects on levodopa-induced dyskinesias in Parkinson’s disease patients: searching for effective cortical targets. Restor Neurol Neurosci, 28(4): 561-568.
INTRODUCTION:
There are many ancient descriptions of Parkinson’s disease, including passages in the Ebers medical papyrus from ancient Egypt, Ayurvedic texts from India, the Bible and the writings of Galen. There were no clear references to parkinsonism in medieval and Renaissance texts, but discussions of parkinsonian symptoms were published by the Dutch anatomist Sylvius and the English surgeon John Hunter during the 17th and 18th centuries.
Quote:The First Described Case and Nomenclature
The first systematic description of paralysis agitans ;was given by the English surgeon, paleontologist and radical politician James Parkinson in an “Essay on the Shaking Palsy” in 1817.
Parkinson described the characteristic rigidity, tremor, hypokinesia and abnormalities of gait and posture in 3 of his own patients and 3 other individuals he had seen on the street.
He correctly established the distinction between resting and motion (intention) tremor that is still recognized today.
But, he was with time found not to be correct in his observation of “the senses and intellect being uninjured” and was wrong in attributing the disorder to a lesion of the cervical spinal cord [1].
Dr. James Parkinson
(source: allaboutparkinsons.com)
Parkinson’s essay prompted communications from a number of British, French and German clinicians, and between 1868 and 1881 Jean-Martin Charcot elucidated the clinical features of several types of paralysis agitans and proposed that the syndrome be named after Parkinson [2].
Time Line of Some Significant Events in the History of Parkinson's Disease
- The association between parkinsonism and dementia in some patients was recognized by the beginning of the 20th century, and characteristic inclusion bodies were described in affected patients by Lewy.
- The brain regions responsible for parkinsonian symptoms, chiefly the basal ganglia and particularly the substantia nigra, were identified by Tretiakoff in 1919, and pathological confirmation was provided by Hassler in 1938.
- The neurochemistry of these centers and the role of dopamine deficiency in parkinsonian symptoms were clarified by Carlsson and Hornykiewicz in the 1950s [3].
- This led in turn to the treatment of these symptoms with dopamine, which was much more successful than previous therapeutic attempts with acetylcholine antagonists and surgical lesioning of the corticospinal tract or basal ganglia structures [4].
- Electrical stimulation of deep brain structures was introduced in the 1990s and has been effective in refractory Parkinon’s disease [5], and recent attention has been focused on the potential regenerative effects of stem cell infusion [6].
CLINICAL FEATURES
Parkinson’s disease has primarily motor symptoms, but since Parkinson’s initial description, non-motor manifestations have been increasingly recognized and may in fact precede motor symptoms.
The motor hallmarks of the disorder are:
a) Bradykinesia or hypokinesia (slowness or reduction of movement)
b) Rigidity (increased muscle tone and resistance to movement due to continuous muscle contraction)
c) Tremor (involuntary rhythmic movement of the limbs when at rest)
d) An abnormal and unstable posture
Non-motor symptoms are numerous and include:
a) Dementia and behavioral or emotional disturbances
b) Autonomic nervous system dysfunction
c) Abnormalities of vision and eye movements [7].
![[Image: parkpic1335108259020.png]](http://classconnection.s3.amazonaws.com/762/flashcards/918762/png/parkpic1335108259020.png)
Source:http://classconnection.s3.amazonaws.com/762/flashcards/918762/png/parkpic1335108259020.png
Digging deep into the Symptoms
MOTOR SYMPTOMS
Effect on Movements
The characteristic posture and slowness of the disorder reflect an increasing number of difficulties in initiating and executing movements with the progression of the disease. Fine movements that are involved in daily activities are commonly affected early, and bradykinesia is the first cause of disability. Some kinds of movement are more prominently affected than others and this varies from patient to patient, as well as being influenced by level of activity and emotional state. Such difficulties can be assessed on neurological examination by performance of rapid alternating movements with hands and feet, and during examination and in day-to-day activities motor performance is often improved by an external cue such as being touched or supported [8].
Muscle Rigidity in Parkinson's Disease
Rigidity reflects an abnormal increase in muscle tone and may be continuous and cause the limb to feel like a “lead pipe” or to be ratchety like the movement of a “cogwheel”. Cogwheel rigidity is thought to be due to the combination of rigidity and tremor. Rigidity usually begins in the neck and shoulders and is asymmetrical, but becomes symmetrical and more generalized with the progression of the disease. Although pain is not usually part of the parkinsonian syndrome, rigid limbs and stiff joints may be painful to patients. The face is often rigid and mask-like, and stiffness of the vocal cords leads to progressive impairment of speaking and swallowing [9].
Tremors in Parkinson's Disease
Tremor is the most apparent symptom, but may be absent in a third of patie, and is usually predominant distally and has a frequency of 4 to 6 Hz. The tremor is present at rest, decreases with movement and disappears during sleep; the circular motion of the hand and fingers resemble the movements formerly used by pharmacists to form pills, and the movement has been called a “pill-rolling” tremor. The tremor plus rigidity will produce characteristic small and tremulous handwriting (micrographia) [10].
Postural Abnormalities in Parkinson's Disease
Postural abnormality is evident early in the disease, with a stooped posture, forward flexion of the trunk when walking and shuffling steps with a tendency to accelerate when walking (festination). Disabling loss of balance is common, with falling occurring in 40 per cent of patients and 10 per cent having weekly falls with a high rate of injury and fracture [11].
NON-MOTOR SYMPTOMS
- Nonmotor symptoms of Parkinson’s disease include daytime sleepiness, nocturnal insomnia and decreased rapid eye movement (REM) sleep.
- The autonomic nervous system is also involved and this can result in orthostatic hypotension, which aggravates postural instability and the consequences of falling, as well as sweating disturbances, constipation and urinary incontinence and characteristic oily skin (seborrhea).
- Impairment of both voluntary and involuntary eye movements can cause blurred or double vision. The major nonmotor problems, however are cognitive and behavioral. Depression, anxiety, apathy and agitation are predominant psychiatric symptoms in that order [12].
- Impulse control disorders, chiefly pathological gambling and eating, hypersexuality and compulsive shopping, have been associated with treated parkinsonism, and are currently ascribed to the effects of dopamine-altering medications [13].
Dementia is an increasingly recognized complication of parkinsonism. The incidence of dementia is increased sixfold in Parkinson’s disease patients compared to others of the same age, and the quality of life for Parkinson’s patients with dementia, and for their caregivers, is significantly worse than for nondemented patients and those who care for them. Mortality and inability to live independently are also significantly increased in the presence of dementia. Cognitive problems are in fact evident early in the course of Parkinson’s disease and affect most parkinsonian patients and increase in severity with duration of the disease. The cognitive problems are mostly executive functions, like planning, initiating appropriate responses and inhibiting inappropriate ones and speed and flexibility of thinking and abstraction. Memory disturbances are dominated by impaired recall of learned information, and like motor slowness the slowness of recall can be improved by external cues [15].
Monitoring Progression of Parkinson's Disease
- The progression of Parkinson’s disease has classically been measured by the five-stage Hoehn and Yahr scale, which extends from minimal symptoms in stage I to bedfast immobility in Stage V.
- The Unified Parkinson’s Disease rating scale (UPDRS) is a more common recent metric, and suggests loss of the ability to ambulate after 8 years of disease and immobility after 10 years if not successfully treated.
Quote:The multiplicity of available treatments has prolonged survival, but progression to requirement for caretakers occurs over about 15 years. Fifty per cent of treated patients have disability from various problems with dopamine-augmenting medications during the first 5 years of treatment, whereas disability is predominantly due to nonmotor symptoms like cognitive decline and autonomic dysfunction after 10 years of treatment. Life expectancy is significantly reduced, and mortality rates are also about three times greater in those with parkinsonism [16].
EPIDEMIOLOGY
- Parkinson’s disease afflicts about 7 million people around the world and around 1 million in the United States, making it the second most common neurodegenerative disorder after Alzheimer’s disease. Its prevalence is around 0.3 per cent in general, 1 per cent at age 60 and 4 per cent in the population over 80 years old, while its incidence is 8 to 18 cases per 100,000.
- Ninety-five per cent have onset around age 60, the remainder (young-onset Parkinson’s disease) beginning usually between 20 and 50 years old. Most studies suggest a predominance in Caucasians, and some also suggest a greater incidence in men.
- Parkinsonism is more common after head injury, and an increase in boxers (dementia pugilistica) is well known and epitomized by longtime heavyweight champion Muhammad Ali. Higher rates of parkinsonism have also been associated with rural domicile and certain chemical exposures, particularly pesticides and aluminium, and lower rates have been reported in cigarette smokers [17].
GENETICS
About 15 per cent of parkinsonian patients have a first-degree relative with Parkinson’s disease, and heredity because of an identifiable genetic mutation has been shown in 5 per cent.
- The best-known mutations involve the SNCA gene that codes for alpha-synuclein, the protein contained in the inclusion bodies described a century ago by Lewy in patients with parkinsonism and dementia.
- Another genetic association involves parkin, a protein involved in the degradation of other proteins that is coded by the PARK2 gene; mutation of this gene produces autosomal recessive Parkinson’s disease of juvenile onset.
- The LLRK2 gene which codes for leucine-rich repeat kinase 2 or dardarin, a name derived from the Basque word for “trembling”, has been linked with accelerated cell death in cortical neurons and with an increased risk for Parkinson’s and Crohn’s diseases, and is the most common cause of familial parkinsonism.
CAUSES
The cause of Parkinson’s disease remains unknown but it is clearly related to dopamine and related neurotransmitters. The chief pathological feature is death of dopamine-containing cells in the substantia nigra in the midbrain, particularly the front or ventral part of the pars compacta, ordinarily a dense collection of cell bodies that is highly pigmented by melanin but in Parkinson’s disease largely depopulated and depigmented, as up to 70 per cent of dopaminergic neurons are gone by the time of death. This brain region has widespread connections through 5 different pathways which involve dopamine neurotransmission, dysfunction of which can produce the characteristic symptoms of Parkinson’s disease: these pathways involve motor cortex, limbic system, orbitofrontal cortex, association cortex and oculomotor projections.
![[Image: parkinsons-disease.jpg]](http://callmyfamilydoctor.com/blog/wp-content/uploads/2015/01/parkinsons-disease.jpg)
Source: callmyfamilydoctor.com
The motor pathway may exert an inhibitory effect on the other motor centers and neurons, and dopamine may act to reduce that inhibition so that appropriate motor activity can take place; high levels of dopamine would be associated with increased motor activity, as is often seen with overtreatment of parkinsonian symptoms, while low levels would be associated with hypokinesia. Projections to the limbic system connecting the midbrain, frontal and temporal lobes may be responsible for psychosis and other mental symptoms, while projections to the frontal lobes and orbitofrontal cortex may play a role in diminished inhibition of inappropriate behavior and impairment of executive functions. Another dopamine projection is to association areas of the cerebral cortex, which could be involved in memory dysfunction, and the disturbances of eye movement and visuospatial perception characteristic of the disease could reflect impaired dopamine input through the oculomotor pathway that connects to eye movement fields in the frontal lobes [19].
The cause of the massive neuronal cell death is not known, but attention is increasingly focused on the inappropriate accumulation of possibly toxic proteins, by analogy with other neurodegenerative disorders. Recent evidence suggests that one or more of several types of accumulated synuclein may cause the cell death, perhaps by an immune mechanism, and that this may be in some cases triggered by environmental exposures and in other cases due to the permissive effect of genetic factors like LLRK-2 deficiency or malfunction. Misfolded and therefore inactive proteins due to virus-like infectious protein particles (prions) that have been linked to “mad-cow” disease, scrapie in sheep and kuru in humans may also trigger parkinsonian neurodegeneration. This has led to promising trials of immunotherapy with monoclonal antibodies and neuroprotection through infusion of stem cells [20].
DIAGNOSIS
Parkinson’s disease is diagnosed clinically by a history of motor and cognitive symptoms and a neurological examination showing tremor, rigidity, bradykinesia and abnormal gait and posture.
- Computed tomography (CT) and magnetic resonance imaging (MRI) are generally normal but may help in excluding alternative causes for symptoms.
- Positron tomography (PET) and single photon emission CT (SPECT) with radioactive iodine or fluorine tracers may show decreased dopamine activity in the basal ganglia [21].
Quote:Clinical diagnostic criteria have been proposed by the Parkinson’s Disease Society Brain Bank in the United Kingdom and the National Institute of Neurological Disorders and Stroke in the United States: these involve excluding other causes for motor symptoms and finding resting tremor, unilateral onset or asymmetric symptoms, progression of symptoms with time, a clinical course of 10 or more years, response to levodopa for at least 5 years or excessive movement (dyskinesia) if given excessive levodopa [22].
TREATMENT
Great advances have been made in the past 50 years in treatment of Parkinson’s disease.
The approaches to treatment may be classified into (a) Medical (b) Surgical
Medical treatment has been aimed at replacement of dopamine, augmentation of its effect at dopamine receptors, impairment of its degradation so as to preserve dopamine effects at the receptors, stabilization of its highly variable effects over time and amelioration of symptoms with drugs that act by different mechanisms.
Medical treatment has been aimed at replacement of dopamine, augmentation of its effect at dopamine receptors, impairment of its degradation so as to preserve dopamine effects at the receptors, stabilization of its highly variable effects over time and amelioration of symptoms with drugs that act by different mechanisms.
Surgical lesioning of pathways responsible for the motor symptoms was supplanted by ablation of subcortical areas involved in them, and subsequently by transcortical magnetic stimulation or the implantation of pacemaker devices to stimulate deep brain structures.
Recent studies of immunotherapy and neuroprotection have also attracted attention.
Medical approaches have focused on dopamine replacement or augmentation. L-DOPA (levodopa) is converted to dopamine by the enzyme DOPA decarboxylase in dopaminergic neurons, and has been used since in 1970s to replace depleted dopamine and alleviate motor symptoms. Since only about 5 per cent of L-DOPA crosses the blood-brain barrier and the remainder causes peripheral symptoms of dopamine excess such as nausea, peripheral DOPA decarboxylase inhibitors such as carbidopa and benserazide have been given along with levodopa or added to combination preparations such as Sinemet, the combination of carbidopa and levodopa which was named from the Latin for “without vomiting”. With increasing duration of treatment, patients may become less responsive to levodopa or have disabling fluctuations between rigidity and hyperkinesis, the “on-off” phenomenon. These were first addressed by “drug holidays”, periods in which levodopa was withdrawn, but opinion has turned against this because dyskinesias may become worse and life-threatening neuroleptic malignant syndrome may occur when the dopamine replacement is restarted.
Adjunctive drugs that inhibit the enzyme catechol-o-methyltransferase (COMT), which breaks down levodopa, may prolong and stabilize the dopaminergic effect. The first such drug, tolcapone, proved to have potentially fatal liver toxicity and has been withdrawn in most countries and is very rarely used in the others. Entacapone does not have the same toxicity, and is used by itself or in a combination preparation with levodopa and carbidopa. Another way to augment the effect of levodopa is to slow the metabolism of the dopamine it is made into by inhibiting monoamine oxidase B (MAO-B). This can be done with the vitamin E derivatives seligiline and rasagaline.
Direct stimulation of the dopamine receptor may be an alternative to levodopa, and have been shown to delay the onset of motor complications like dyskinesias and “on-off”. Bromocriptine, pergolide, pramipexole, ropinirole and cabergoline, along with piribedil outside the United States, can be taken orally, while apomorphine is given by injection and two receptor agonists (lisuride and rotigotine) can be administered through skin patches. These are increasingly used in the attempt to avoid levodopa early in the disease and to lessen motor complications later.
Surgical treatments began with lesions of the corticospinal tract to control tremor. With the introduction of the anticholinergic drugs such procedures declined, and lesions were occasionally made in selected motor areas in refractory patients, chiefly to diminish disabling dyskinesias. Pallidotomy, the cauterization of a small part of the globus pallidus, which is adjacent to the substantia nigra and through which most of its motor nerve output passes, has been the most common lesional operation for the combination or rigidity, tremor and dyskinesia. Lesioning of the thalamus (thalamotomy) with electrical cauterization or the application of a supercooled probe has also been carried out, particular for severe tremor, and has mainly focused on the subthalamic nucleus on the side opposite to the tremor. The operations are usually done under local anesthesia, as the brain itself is insensitive to pain, and the effect of the lesion on motor symptoms can be immediately assessed. The complications are those of craniotomy, chiefly bleeding and infection.
Deep Brain Stimulation grew out of lesioning surgeries, and was approved in the United States for tremor in 1997 and Parkinson’s disease in 2002, as well as for dystonia, obsessive-compulsive disorder, refractory depression and chronic pain. An implantable pulse generator in titanium housing is placed below the clavicle or in the abdomen, and is connected to a polyurethane-insulated coiled wire with four electrodes made of platinum and iridium, that are placed in one or two target nuclei. The electrodes are placed in the globus pallidus or subthalamic nucleus. The implantation is often done under general anesthesia with the device and the target nuclei visualized by intraoperative MRI, after which the effects of stimulation on tremor and dyskinesia can be calibrated when the patient is awake. The potential risks are again infection, estimated at 3 to 5 per cent, and hemorrhage in 1 or 2 per cent. There has also been interest in noninvasive brain stimulation through the skull with the transcortical magnetic device approved for refractory depression and migraine; some improvement in levodopa-related dyskinesia has been reported [23].
![[Image: 161px-Parkinson_surgery.jpg]](http://upload.wikimedia.org/wikipedia/commons/thumb/9/91/Parkinson_surgery.jpg/161px-Parkinson_surgery.jpg)
Electrode insertion during deep brain stimulation (Source: wikimedia)
New developments include promising attempts at treatment derived from the evidence that the accumulation of proteins normally disposed of may be harmful or fatal to the neurons of the substantia nigra by inciting an immune response, by triggering the processes of cell death that are programmed into all tissues or by the lack or loss of trophic factors or substances that protect neurons against this process. After promising studies in which monoclonal antibodies were targeted with beneficial effect against beta-amyloid, the protein whose inappropriate accumulation may be responsible for Alzheimer’s disease, studies are underway of passive and active immunization against the alpha-synuclein which accumulates in neurons in parkinsonism with apparent fatal effect. Active immunization involves injecting a small fraction of synthetic alpha-synuclein to induce the formation of antibodies, while passive immunization involves the preparation of monoclonal antibodies against alpha-synuclein which are then infused. The third approach is similar to recent advances in the treatment of amyotrophic lateral sclerosis, and involves transforming pluripotent adult stem cells into cells capable of secreting nerve growth factors known to be protective against cell death, then reinfusing these stem cells into the patient to offer neuroprotection to the imperiled dopaminergic neurons. These therapeutic options are now approaching or beginning phase 2 trials in hopes of demonstrating efficacy against Parkinson’s disease [20].
REFERENCES
1. Wilkins RH, Brody IA (1997). Neurological Classics. New York, Thieme. Pp. 87-92.
2. Lees AJ (2007). Unresolved issues related to the shaking palsy on the celebration of James Parkinson’s 250th birthday. Movement Disord, 22(suppl 17): S327-334.
3. Fahn S (2008). The history of dopamine and levodopa in the treatment of Parkinson’s disease. Movement Disord, 23(suppl 3): S497-508.
4. Lanska DJ (2010). The history of movement disorders. Handbook Clin Neurol, 95: 501-546.
5. Coffey RJ (2009). Deep brain stimulation devices: a brief technical history and review. Artif Organs, 33(3): 208-220.
6. Pollitis M, Lindvall O (2012). Clinical application of stem cell therapy in Parkinson’s disease. BMC Medicine, 10: 1.
7. Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA (2009). Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol, 8(12): 1128-1139.
8. Jankovic J (2008). Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiat, 79(4): 368-376.
9. Fung VS, Thompson PD (2007). Rigidity and spasticity. In, Tolosa E, Jankovic J (eds). Parkinson’s Disease and Movement Disorders. Hagerstown MD, Lippincott Williams & Wilkins, pp. 504-513.
10. Cooper G, Eichhorn G, Rodnitzky RL (2008). Parkinson’s disease. In, Conn PM (ed). Neuroscience in Medicine. Totowa NJ, Humana Press, pp. 508-512.
11. Yao SC, Hart AD, Terzella MJ (2013). An evidence-based osteopathic approach to Parkinson’s disease. Osteopath Fam Phys, 5(3): 96-101.
12. Murray ED, Buttner EA, Price BH (2012). Depression and psychosis in neurological practice. In, Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds). Bradley’s Neurology in Clinical Practice, ed. 6. Philadelphia, Elsevier-Saunders, pp. 102-103.
13. Ceravolo R, Frosini D, Rossi C, Bonuccelli U (2009). Impulse control disorders in Parkinson’s disease: definition, epidemiology, risk factors, neurobiology and management. Parkinson Relat Disord, 15(suppl 4): S111-115.
14. Friedman JH (2010). Parkinson’s Disease psychosis 2010: A review article. Parkinson Relat Disord, 16(9): 553-560.
15. Caballol N, Martí MJ, Tolosa E (2008). Cognitive dysfunction and dementia in Parkinson disease. Movement Disord, 22 (suppl 17): S358-366.
16. Poewe W (2006). The natural history of Parkinson’s Disease. J Neurol, 253(7 suppl): vii2-vii6.
17. Wright-Willis A, Evanoff BA, Lian M (2010). Geographic and ethnic variation in Parkinson disease: a population-based study of U.S. Medicare beneficiaries. Neuroepidemiol, 34: 143-151.
18. Shulman JM, De Jager PL, Feany MB (2011). Parkinson’s Disease: genetics and pathogenesis. Ann Rev Pathol, 6: 193-222.
19. Sontheimer H (2015). Diseases of the Nervous System. Waltham, MA, Elsevier. Pp. 134-166.
20. Stetka BS, Facheris M (2015). Prions, the pipeline and the latest in Parkinson’s disease. Medscape Neurology, May 8, 2015.
21. Brooks DJ (2010). Imaging approaches to Parkinson’s Disease. J Nucl Med, 51(4): 596-609.
22. Lingor P, Liman J, Kallenberg K, Sahlmann C-O, Bähr M (2011). Diagnosis and differential diagnosis of Parkinson’s Disease. In, Rana AQ (ed). Diagnosis and Treatment of Parkinson’s Disease. Rijeka, Croatia, InTech,com, pp. 1-20.
23. Koch G (2010). rTMS effects on levodopa-induced dyskinesias in Parkinson’s disease patients: searching for effective cortical targets. Restor Neurol Neurosci, 28(4): 561-568.
by SunilNagpal at 05-11-2015, 10:39 PM
6 comments
Acne vulgaris, or just Acne, is a fairly common, but a painful and often stressful disease, which is faced by most of the 'young ones' (especially adolescents). Owing to its affect on 'the face' of a person, it not only causes the pain associated with the disease, but also leads to anxiety, stress, lowered self-esteem or even suicides (in extreme cases). This article has thus been initiated to provide information about the disease from causes to cure, apart from being a platform for discussions related to Acne.
![[Image: 230px-Akne-jugend.jpg]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/4b/Akne-jugend.jpg/230px-Akne-jugend.jpg)
An indicative image of Acne (courtesy: wikimedia)
| NOTE |
What exactly Acne is?
Acne (or pimples) are result of the blockage of the hair follicles (pores in the human skin: can be observed through a magnifying glass), which are actually connected to oil glands (sebaceous glands) located underneath the skin. An oily liquid, called sebum, is produced by these glands. Apart from hairs growing out of the follicle, sebum carries out the dead skin cells on the surface. It's when these follicles get blocked that a pimple/acne develops (leading to an accumulation of sebum under the skin). Bacteria tend to grow/infect the blocked follicles filled with keratin and lipid making the condition even worse.
![[Image: 515_Acne_formation.jpg]](http://upload.wikimedia.org/wikipedia/commons/3/34/515_Acne_formation.jpg)
Types of Acne
Often people live with the ignorance that "those red-rashy-puss filled" bumps on the face are typical acne! But the truth is that Acne are of various types; and in fact, some of the most common beauty loop-holes associated with face 'are actually acne' only. Even Blackheads and whiteheads are also two types of Acne!
Following are the various types of acne:
a) Blackheads
b) Whiteheads
c) Papules & Pustules
d) Back Acne
e) Acne conglobata
Blackheads
Blackheads are one of the most common types of Acne faced by innumerable people across the globe. Also called as open comedones, blackheads are created out of plugging of the hair follicle and subsequent pushing of the sebum through to the surface. This oil/sebum turns black in color, upon exposure to the air, and buildup of melanin-skin’s dark pigment.
Blackheads are mostly caused to those people whose skin poduces excess of oil.
![[Image: blackhead.jpg]](http://www.acneskincare.tips/wp-content/uploads/2014/10/blackhead.jpg)
Whiteheads
White heads are also amongst the most common type of Acne that affect almost everyone (once or manytimes in life). Also called as closed comedones, whiteheads appear on one's skin in the form of a white/creamy bump. As you must have understood from the name (closed comedones), whiteheads appear when the clogged sebum is not exposed to the air and remains trapped in the plugged follicle.
Papules and Pustules
Papules and pustules are painful/inflammatory forms of acne, which develop when the walls of the clogged pores end up breaking out of severity/irritation.
Papules appear as closed, red small bumps surrounded by skin inflammation on the face of a person. Unlike blackheads they reveal no visible pores, neither are they white like whiteheads. They are hard/beady when you touch them.
Pustules are actually those typical pimples that scar one's face. They are severe form of papules. These are mid-sized bumps on the face with marked white-yellow dot (pus/fluid) in the center of it. Inflammation is also associated with them.
Acne conglobata (AC)
Acne conglobata (AC) is the most severe form of acne. It is highly severe, but it is rare. But when it happens, it can affect your chest, buttocks, back, shoulders, thighs, upper arms and even your face.
AC is characterized by burrowing and interconnecting abscesses on affected region with irregular scars (both keloidal and atrophic). It is highly inflammatory (hence painful) and also leads to significant disfigurement of the region. Cysts and nodules are formed (comedones), with foul smelling seropurulent material. They have the tendency to reappear even after drainage of the material from cysts.
A sudden deterioration of existing active papules/pustules; or recrudescence of acne dormant for many years can cause AC.
How to Avoid Acne? Tips for Skin Care
Acne has a genetic influence, but it is often triggered by following environmental stresses:
![[Image: 230px-Akne-jugend.jpg]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/4b/Akne-jugend.jpg/230px-Akne-jugend.jpg)
An indicative image of Acne (courtesy: wikimedia)
| NOTE |
If you voted No to the poll above (i.e you never got any acne in life), then congratulations for having some out of the world genetic make-up! Because, almost every individual on this earth has once/many a times in life, suffered from Acne in one form or the other!
What exactly Acne is?
Acne (or pimples) are result of the blockage of the hair follicles (pores in the human skin: can be observed through a magnifying glass), which are actually connected to oil glands (sebaceous glands) located underneath the skin. An oily liquid, called sebum, is produced by these glands. Apart from hairs growing out of the follicle, sebum carries out the dead skin cells on the surface. It's when these follicles get blocked that a pimple/acne develops (leading to an accumulation of sebum under the skin). Bacteria tend to grow/infect the blocked follicles filled with keratin and lipid making the condition even worse.
![[Image: 515_Acne_formation.jpg]](http://upload.wikimedia.org/wikipedia/commons/3/34/515_Acne_formation.jpg)
Events leading to Acne (courtesy: wikimedia)
Types of Acne
Often people live with the ignorance that "those red-rashy-puss filled" bumps on the face are typical acne! But the truth is that Acne are of various types; and in fact, some of the most common beauty loop-holes associated with face 'are actually acne' only. Even Blackheads and whiteheads are also two types of Acne!
Following are the various types of acne:
a) Blackheads
b) Whiteheads
c) Papules & Pustules
d) Back Acne
e) Acne conglobata
Blackheads
Blackheads are one of the most common types of Acne faced by innumerable people across the globe. Also called as open comedones, blackheads are created out of plugging of the hair follicle and subsequent pushing of the sebum through to the surface. This oil/sebum turns black in color, upon exposure to the air, and buildup of melanin-skin’s dark pigment.
Blackheads are mostly caused to those people whose skin poduces excess of oil.
Quote:It's thus a huge myth that blackheads are caused by dirt, the truth is that they are Acne; and are a result of oxidation of the oil pushed out of plugged follicles & buildup of melanin.
![[Image: blackhead.jpg]](http://www.acneskincare.tips/wp-content/uploads/2014/10/blackhead.jpg)
Source:http://www.acneskincare.tips/wp-content/uploads/2014/10/blackhead.jpg
Whiteheads
White heads are also amongst the most common type of Acne that affect almost everyone (once or manytimes in life). Also called as closed comedones, whiteheads appear on one's skin in the form of a white/creamy bump. As you must have understood from the name (closed comedones), whiteheads appear when the clogged sebum is not exposed to the air and remains trapped in the plugged follicle.
Papules and Pustules
Papules and pustules are painful/inflammatory forms of acne, which develop when the walls of the clogged pores end up breaking out of severity/irritation.
Papules appear as closed, red small bumps surrounded by skin inflammation on the face of a person. Unlike blackheads they reveal no visible pores, neither are they white like whiteheads. They are hard/beady when you touch them.
Pustules are actually those typical pimples that scar one's face. They are severe form of papules. These are mid-sized bumps on the face with marked white-yellow dot (pus/fluid) in the center of it. Inflammation is also associated with them.
Back Acne
Our back possesses a large number of oil and sweat glands. Back acne thus appear when the follicles leading to the surface for oil secretions get blocked. Puberty is often considered to be the active age for back acne with augmented symptoms in early twenties. Back acne aren't common in adults (post 20 years), though they can appear in some cases.
Trapping of heat/sweat in the body; and aberrations to skin due to friction are also known to cause back acne.
![[Image: How-to-Get-Rid-of-Back-Acne-1.jpg]](http://learntogetridof.com/wp-content/uploads/2015/01/How-to-Get-Rid-of-Back-Acne-1.jpg)
Our back possesses a large number of oil and sweat glands. Back acne thus appear when the follicles leading to the surface for oil secretions get blocked. Puberty is often considered to be the active age for back acne with augmented symptoms in early twenties. Back acne aren't common in adults (post 20 years), though they can appear in some cases.
Trapping of heat/sweat in the body; and aberrations to skin due to friction are also known to cause back acne.
![[Image: How-to-Get-Rid-of-Back-Acne-1.jpg]](http://learntogetridof.com/wp-content/uploads/2015/01/How-to-Get-Rid-of-Back-Acne-1.jpg)
A typical case of back-acne
Acne conglobata (AC)
Acne conglobata (AC) is the most severe form of acne. It is highly severe, but it is rare. But when it happens, it can affect your chest, buttocks, back, shoulders, thighs, upper arms and even your face.
AC is characterized by burrowing and interconnecting abscesses on affected region with irregular scars (both keloidal and atrophic). It is highly inflammatory (hence painful) and also leads to significant disfigurement of the region. Cysts and nodules are formed (comedones), with foul smelling seropurulent material. They have the tendency to reappear even after drainage of the material from cysts.
A sudden deterioration of existing active papules/pustules; or recrudescence of acne dormant for many years can cause AC.
How to Avoid Acne? Tips for Skin Care
Acne has a genetic influence, but it is often triggered by following environmental stresses:
- Oily skin products like moisturizers/cosmetics can cause acne, so choose the cosmetics carefully.
- Oils/Fats/Grease encountered in kitchen/work.
- Pressure equipment like Sports helmets that are too tight. Tight clothes can also cause acne.
- Pollution and high humidity are also major contributors.
- Avoid sun-tan/sun-burns.
- Squeezing should be avoided.
- Avoid hard scrubbing of skin.
- Avoid Anxiety/Stress! (a single major contributor to all major diseases!!)
Article Under Update
by Dipankur Gautam at 05-10-2015, 06:22 PM
0 comments
Dear Sir/Madam,
Warm greeting from IABSciences. IABSciences is one of the leading Animal Biotechnology Company in north India.We are the sole manufacturer of Animal Tissue Culture and Animal Biotechnology teaching kits.These Kits are very useful in Biotechnology universities/Colleges and Research Institutes.
We work on different Cancer cell lines.In this, we analyse and check the effect of anti cancer drugs.
Our expertise in Animal Cell/Tissue Culture and in Stem Cell Technology.
Apart from this,We provide Dissertation/Research Work/ Training on the followings areas:
ANIMAL CELL/TISSUE CULTURE
STEM CELL TECHNOLOGY
MOLECULAR BIOLOGY
MICRO BIOLOGY
IMMUNO-TECHNOLOGY
REPRODUCTIVE BIOTECHNOLOGY
WORKING ON DIFFERENT CELL LINES
For more information you can visit our web site :www.iabsciences.com
We have very limited number of seats for June-July batch.
Our registration window is open , if you are interested you can apply online ASAP.
Thanks and Regards,
IABSciences
www.iabsciences.com
Warm greeting from IABSciences. IABSciences is one of the leading Animal Biotechnology Company in north India.We are the sole manufacturer of Animal Tissue Culture and Animal Biotechnology teaching kits.These Kits are very useful in Biotechnology universities/Colleges and Research Institutes.
We work on different Cancer cell lines.In this, we analyse and check the effect of anti cancer drugs.
Our expertise in Animal Cell/Tissue Culture and in Stem Cell Technology.
Apart from this,We provide Dissertation/Research Work/ Training on the followings areas:
ANIMAL CELL/TISSUE CULTURE
STEM CELL TECHNOLOGY
MOLECULAR BIOLOGY
MICRO BIOLOGY
IMMUNO-TECHNOLOGY
REPRODUCTIVE BIOTECHNOLOGY
WORKING ON DIFFERENT CELL LINES
For more information you can visit our web site :www.iabsciences.com
We have very limited number of seats for June-July batch.
Our registration window is open , if you are interested you can apply online ASAP.
Thanks and Regards,
IABSciences
www.iabsciences.com
by Anupama at 05-09-2015, 04:53 PM
2 comments
I have completed my M.Tech in Food Biotechnology Engineering and qualified GATE BT 2015 with air 701 and score 427. I got a call for Ph.D interview from Kusuma School Of Biological Sciences, IIT Delhi. I am very interested to do my Ph.D from reputed institute but I am facing problems in searching research areas regarding Food Biotech. I have searched facuty profiles of IIT Delhi but I couldn't find any research interest related to my M.tech thesis ( Probiotics). I don't understand what will I do during interview when they ask me for research interest? If I prepare my research proposal according to any professor's research interest (like study of microbial products having therapeutic use for humans, I am intersted in this topic also)then will they consider me for admission despite of having thesis work on different topic. I am really very confused about this matter and I don't have anyone for guidance. If I want to go for NUS, is there any possibility for me? Is it fine in a financial point of view ( is it costly to do Ph.D outhere)?
I have read your previous suggestions and your stories and I really found them very helpful. You are doing a great job. Please help me also.
I have read your previous suggestions and your stories and I really found them very helpful. You are doing a great job. Please help me also.
by ssb20 at 05-09-2015, 12:20 AM
3 comments
Hello all,I just wanted to know if there is any way to get answers that are given at the end of chapters
I need ans for Cell Biology book by Gerald Karp
For lehningers it is there online...But I couldn't find online for karp...
Thanks in advance
I need ans for Cell Biology book by Gerald Karp
For lehningers it is there online...But I couldn't find online for karp...
Thanks in advance
by bmanna at 05-08-2015, 01:58 PM
20 comments
Received call letter for interview in MSR from School of biological sciences, IIT-Delhi (http://bioschool.iitd.ac.in/ ). Please give preparatory suggestions and valuable advices to face the interview.
Regards,
Bharat.
Regards,
Bharat.
by RajniSahni at 05-07-2015, 08:08 PM
2 comments
I have a curious question (not asked in any assignment). I have always wondered as to why Mosquito bite doesn't cause AIDS? What if a Mosquito bites an AIDS patient and then bites a healthy person? The blood carried by the mosquito won't transfer the virus? I know it is established that mosquito bite can't cause AIDS, but can anyone help me in getting my concept cleared about why it is not possible ?
Looking forward to your response.
Thanks
Rajni
Looking forward to your response.
Thanks
Rajni
by Miles E. Drake at 05-07-2015, 02:53 PM
4 comments
Amyotrophic lateral sclerosis (ALS) is a progressive and generally fatal neurological disorder of upper and lower motor neurons, causing weakness, muscle wasting, abnormal muscle contractions (fasciculations) and altered muscle tone and reflexes. Emotional or cognitive disturbances rarely occur. The cause is not yet known, although a few potential causative factors have been identified and a small percentage of cases appear to have a genetic basis.
CLINICAL FEATURES
The diagnosis is made by history of weakness and muscle wasting, and by the finding on neurological examination of diminished strength, muscle atrophy, fasciculations in the limb or trunk muscles, the combination of atrophy and fasciculations in the tongue and hyperactive deep tendon and jaw jerk reflexes with evidence of spasticity such as Babinski’s sign.
Fasciculations in the EMG are especially ominous, and the EMG is also commonly obtained to exclude muscle disease, while nerve conduction velocities are also measured and are normal in ALS but slowed in peripheral nerve disorders. MRI and other imaging studies are likewise normal, but may be obtained to exclude lesions of the brain stem or cervical spinal cord that can mimic these symptoms [4].
EPIDEMIOLOGY
GENETICS
Between 5 and 10 per cent of ALS cases are apparently familial, and first-degree relatives of ALS patients have a risk of about 1 per cent for developing the disease [9]. The most common familial forms of ALS are associated with a series of autosomal dominant mutations of a gene on chromosome 21 coding for the enzyme superoxide dismutase that is prominently involved in antioxidant protection of tissues from toxic free radicals. These comprise about 20 per cent of familial ALS cases, and represent about 2 per cent of all ALS patients. The SOD1 mutation is associated with rapidly progressive ALS and has been described in North American cases; Scandinavian patients have been described with a slightly different mutation called D90A-SOD1, and have a more indolent disease lasting on average 11 years from onset to death. These mutations may result in an ineffective antioxidant enzyme and accumulation of free radicals with resultant motor neuron damage [10].
CAUSES OF ALS (Amyotropic Lateral Sclerosis)
TREATMENT OF ALS (Amyotropic Lateral Sclerosis)
CONTRIBUTIONS OF BIOTECHNOLOGY
1. Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011). Amyotrophic lateral sclerosis. Lancet, 377(9769): 42-55.
2. Turner MR, Swash M, Ebers GC (2010). Lockhart Clarke’s contribution to the description of amyotrophic lateral sclerosis. Brain, 133(11): 3470-3479.
3. Hardiman O, van den Berg LH, Kiernan MC (2011). Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol, 7(11): 639-649.
4. Brooks BR, Miller RG, Swash M, Munsat TL (2000). El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler, 1(5): 293-299.
5. Eisen A (2009). Amyotrophic lateral sclerosis: A 40-year personal perspective. J Clin Neurosci, 16(4): 505-512.
6. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz M (2010). Clinical significance of the change of decline in ALSFRS-R. Amyotroph Lateral Scler, 11(1-2): 178-180.
7. Turner MR, Parton MJ, Shaw CE, Leigh PN, Al-Chalabi A (2003). Prolonged survival in motor neuron disease: a descriptive study of the King’s database, 1990-2002. J Neurol Neurosurg Psychiat, 74(7): 995-995.
8. Stephen Hawking serves as a role model for ALS patients. CNN, April 21, 2009.
9. Sontheimer H (2015). Diseases of the Nervous System. Waltham, MA, Academic Press, pp. 165-172.
10. Battistini S, Ricci C, Lotti EM, Benigni M, Gagliardi S, Zucco R, Bondavalli M, Marcello N, Ceroni M, Cereda C (2010). Severe familial ALS with a novel exon 4 mutation (L106F) in the SOD1 gene. J Neurol Sci, 293(1): 112-115.
11. DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R (2011). Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9-linked FTD and ALS. Neuron, 72(2): 245-256.
12. Deng H-X, Chen W, Hong S-T, Boykott K, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, Jiang H, Hirano M, Rampersaud E, Jansen GH, Dankervoort S, Bigio H, Brooks BR, Ajroud K, Sufit RL, Haines JL, Mugnaini E, Pericak-Vance MA, Siddique T (2011). Mutations in UBQLN2 gene cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature, 477: 211-215.
13. Al-Chalabi A, Leigh PN ( 2000). Recent advances in amyotrophic lateral sclerosis. Current Opinion in Neurology, 13(4): 397–405.
14. Manuel M, Heckman CJ ( 2011). Stronger is not always better: could a bodybuilding dietary supplement lead to ALS? Exp Neurol, 228(1): 1-5.
15. Rodgers KJ ( 2014). Non-protein amino acids and neurodegeneration: The enemy within. Exp Neurol, 252(3): 192-196.
16. Miller RG, Mitchell JD, Lyon M, Moore DH (2007). Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database System Rev, 1: CD001447.
17. Li Y, Balasubramanian U, Cohen D, Zhang P-W, Mosmiller E, Sattler R, Maragakis NJ, Rothstein JD (2015). A Comprehensive Library of Familial Human Amyotrophic Lateral Sclerosis Induced Pluripotent Stem Cells. PLOS One, 10(3): e011826. doi 10.1371/journal.pone.0118266.
Related images from the web
![[Image: 224239main_hawkingImage-13.jpg]](http://www.nasa.gov/images/content/224239main_hawkingImage-13.jpg)
![[Image: ei_2709.jpg]](http://www.uchospitals.edu/images/gs/ei_2709.jpg)
Source: htttp://www.uchospitals.edu/images/gs/ei_2709.jpg
The diagnosis is customarily made clinically and by exclusion of some other conditions, but some abnormalities of the electromyogram (EMG) are also characteristic. Treatment is largely ineffective, and attention is mainly directed toward management of the various symptoms; most patients die of respiratory complications within 5 years and almost all within 10 years. There have been many high-profile ALS cases, including baseball great Lou Gehrig, whose name is often given to the disease, and there are a few noted examples of long survival, chiefly theoretical physicist Stephen Hawking [1].
Quote:
- The disease has also been named after Jean-Martin Charcot, who first described it comprehensively as with many other neurological disorders.
- The first clinical observation of probable ALS, emphasizing weakness and wasting, was made by Charles Bell in 1836, and Augustus Waller described the degeneration of motor nerve fibers that came to bear his name in 1850.
- The condition was at first called progressive muscular atrophy, a term also applied to various muscle and peripheral nerve disorders, and a similar condition but with upper motor neuron features like spasticity and increased reflexes was identified in the 1860s.
- Charcot differentiated a disease of motor neurons from other neuromuscular disorders in 1874, and introduced the name the disease now carries.
- The first comprehensive neurological textbooks, by Gowers in English and Oppenheim in German, suggested that ALS cases could involve upper or lower motor neurons, or both, with different symptom profiles, and the term “motor neuron disease” that is now used interchangeably with ALS was suggested for this complex of symptoms by Sir Russell (later Lord) Brain in 1933 [2].
CLINICAL FEATURES
- The cardinal manifestations of ALS are muscular weakness and atrophy, beginning with clumsiness, impaired fine motor skills, stiffness or aching of an arm or leg in about 75 per cent of patients (limb-onset).
- Bulbar (brainstem) onset occurs in 25 per cent of cases, with slurred and nasal speech, impaired articulation and difficulty swallowing.
- About 3 per cent of patients have early involvement of the intercostal muscles and experience early breathing difficulties along with other symptoms (respiratory onset).
- Cognitive changes can be found with careful interview or neuropsychological testing in 30 to 50 per cent, although only about 5 per cent of patients develop frontotemporal dementia (formerly called Pick’s disease) with cognitive impairment, difficulty with expressive and receptive language, apathy and blunted emotions and sometimes irascible or inappropriate behavior. Sensation and autonomic nervous system function are almost always spared [3].
The diagnosis is made by history of weakness and muscle wasting, and by the finding on neurological examination of diminished strength, muscle atrophy, fasciculations in the limb or trunk muscles, the combination of atrophy and fasciculations in the tongue and hyperactive deep tendon and jaw jerk reflexes with evidence of spasticity such as Babinski’s sign.
Quote:Fasciculations are localized contraction and relaxation of muscles that can be seen under the skin and that represent the discharge of individual motor neurons and the muscle fibers they activate; there are many causes for fasciculations, most of them benign, but widespread fasciculations and in particular involvement of the tongue are indicative of motor neuron disease.
Fasciculations in the EMG are especially ominous, and the EMG is also commonly obtained to exclude muscle disease, while nerve conduction velocities are also measured and are normal in ALS but slowed in peripheral nerve disorders. MRI and other imaging studies are likewise normal, but may be obtained to exclude lesions of the brain stem or cervical spinal cord that can mimic these symptoms [4].
- The disease is progressive but the symptoms are variable, and patients who have bulbar symptoms, lower motor neuron features such as decreased muscle tone and reflexes, respiratory involvement and frontotemporal dementia at onset progress more rapidly.
- Spasticity, hyperactive reflexes and other upper motor neuron features may predict slower progression, as may symptom onset before age 40, symptoms confined to one limb or being slightly overweight.
- The extraocular muscles, which have motor units which are much smaller and more numerous than the other skeletal muscles, are not involved until late in the disease course, probably because of the greater supply of motor neurons; as a result, eye movements may be preserved when other motor function is lost.
- Most patients lose the function of arms and legs and generally speech as well, and will eventually require a feeding tube to avoid aspiration of food and a ventilator on account of respiratory muscle failure [5].
Quote:In addition to Lou Gehrig, a number of athletes have developed ALS, including American football player Steve Gleason and British footballer Don Revie, fitness expert Augie Nieto and a later New York Yankee, “Catfish” Hunter.
Actor Sir David Niven was also a highly fit competitive sailor, and many musicians noted for dexterity died of ALS, including composer Dmitri Shostakovich, rock guitarists Dan Toler and Mike Porcaro and jazz bassist Charles Mingus.
These and other cases have suggested a relationship between physical fitness or agility and ALS risk. Noted political figures with ALS have included New York senator Jacob Javits and Chinese leader Mao Zedong.
The most prominent celebrity case of ALS, however, is physicist Stephen Hawking, who became one of the world’s best-known scientists despite inexorably progressive ALS of very atypically long duration, and who has pioneered the use of multiple adaptive technologies to continue working despite profound neurological deficit. Hawking’s advocacy and widely-noted example has contributed to the popularity of the “ice bucket challenge” as a fund-raising device for ALS research [8].
EPIDEMIOLOGY
ALS is estimated to affect 1 to 3 individuals per 100,000 in the United States Caucasian population, with approximately 5,500 new cases diagnosed annually and about 30,000 active cases. In Europe the disease affects approximately 2.2 individuals per 100,000 per year, predominating among Caucasians. The disease is infrequent among non-Caucasians. Occasional clusters of ALS have been encountered, in the United States in New Hampshire, Vermont, Northeastern Ohio, and Texas among other places, and in several former San Francisco 49er football players. Several proximate cases were also found among Italian and British footballers, and there have been several reports of conjugal ALS affecting husbands and wives in France and Italy, in addition to the small percentage of ALS cases that are hereditary [1].
GENETICS
Between 5 and 10 per cent of ALS cases are apparently familial, and first-degree relatives of ALS patients have a risk of about 1 per cent for developing the disease [9]. The most common familial forms of ALS are associated with a series of autosomal dominant mutations of a gene on chromosome 21 coding for the enzyme superoxide dismutase that is prominently involved in antioxidant protection of tissues from toxic free radicals. These comprise about 20 per cent of familial ALS cases, and represent about 2 per cent of all ALS patients. The SOD1 mutation is associated with rapidly progressive ALS and has been described in North American cases; Scandinavian patients have been described with a slightly different mutation called D90A-SOD1, and have a more indolent disease lasting on average 11 years from onset to death. These mutations may result in an ineffective antioxidant enzyme and accumulation of free radicals with resultant motor neuron damage [10].
Another mutation associated with ALS is a C9orf72, a hexanucleotide repeat on chromosome 9 that has been found in about 6 per cent of European ALS cases, almost all of them with frontotemporal dementia. The mutation has also been found in ALS cases in the Phillipines, and Filipinos are third behind white Americans and Europeans in ALS incidence. The mutation consists of hundreds or repetitions of the nucleotide sequence GGGGCC, which is normally repeated only a few times, and which when excessively repeated may interfere with the normal expression of some protein involved in nerve function [11].
There are many other mutations associated with familial ALS, particularly ALS plus dementia. These cases are often dominantly inherited and linked to the X-chromosome, and are sometimes juvenile in onset, whereas the vast majority of ALS otherwise occurs in middle age and after. The UBQLN2 gene on the X-chromosome ordinarily codes for ubiquilin 2, a protein involved in the breakdown of proteins in motor neurons, and mutation there is thought to result in a nonfunctional gene product that allows the accumulation of harmful damaged proteins [12]. The several other mutations have to date been described only in single families or individuals with ALS, and in all of these cases it is not yet clear what gene product or products are being interfered with to incite the motor neuron degeneration.
CAUSES OF ALS (Amyotropic Lateral Sclerosis)
The cause of amyotrophic lateral sclerosis is unknown, but the etymology of its name, meaning in Greek no muscle nourishment, reflects the traditional belief that some trophic or nourishing factor for motor neurons is absent or becomes deficient. More recent suggestions have involved the accumulation of toxic factors or cell breakdown products, as is becoming apparent in some of the dementias that sometimes coexist with ALS. The chief anatomical feature is death of upper and lower motor neurons in the spinal cord, brain stem and cerebral cortex, preceded by the accumulation in the cell bodies and axons of inclusions, aggregates of proteins that are normally removed or destroyed. Several proteins have been shown to accumulate in ALS: superoxide dismutase, TAR (transactive response) DNA binding protein (TARDBP) and FUS, which is short for RNA binding protein FUSed in sarcoma/translocated in sarcoma. Superoxide dismutase is a protective antioxidant enzyme, while TARDBP and FUS normally stabilize RNA and regulate its transcription in cells but have been shown to accumulate in neurons in frontotemporal dementia and the dementia associated with parkinsonism. These accumulated proteins generally have ubiquitin attached to them; ubiquitin, discovered in 1975 and found almost everywhere in the nervous system (i.e., ubiquitously, hence its name) is a regulatory protein attached to other proteins that are earmarked to be broken down and cleared from the cell. It is likely that these proteins have been tagged for disposal but the protein breakdown system has failed to dispose of them. As a result they accumulate and eventually kill the motor neuron, and when one-third of the motor neurons have been destroyed the symptoms of ALS begin to appear [9].
It is not known why protein disposal fails, but the genetic findings described above could explain the coexistence of familial ALS and dementia. In the other 90 per cent of cases, there is evidence of an increase in blood and spinal fluid of the excitatory neurotransmitter glutamate, which may be toxic to motor neurons and which is reduced by riluzole, the only drug to date to have even minimal effect on the disease13. Branched-chain amino acids, present in the diet and also a common dietary supplement, have been suggested to cause motor neuron hyperexcitability and calcium absorption, which can lead to cell death14. Prions, small proteins that can be transmitted between people and animals as viruses are, can cause neurological disease by interfering with the folding of major cellular proteins and rendering them inactive, and have been implicated in bovine spongiform encephalopathy (“mad cow” disease), Jakob-Creutzfeldt disease and several other conditions; prion-induced protein misfolding has also been suggested as a cause of ALS [15].
TREATMENT OF ALS (Amyotropic Lateral Sclerosis)
The only medication approved for ALS in the United States and United Kingdom is riluzole, a glutamate antagonist which modestly but significantly lengthened survival time after diagnosis, particularly with the brainstem and lower motor neuron signs and symptoms that are prognostically negative. It does not improve the disability or restore damaged or dead motor neurons, however, and liver toxicity must be watched for [16]. The various medical treatments for spasticity and pain are appropriate, amantadine has been used for fatigue and anticholinergic drugs such as older antiparkinsonian agents and tricyclic antidepressants will reduce saliva and lessen the risk of aspiration [5].
Most therapeutic trials for ALS have been discontinued early on account of lack of efficacy or adverse effects. Recent trials of adult stem cells by Israeli investigators have shown not only prolongation of survival but also slowing of the rate of disease progression as measured by the ALSFRS-R and a strong effect on the rate at which lung function declined, which is the major determinant of ALS mortality. The stem cells are reprogrammed into astrocyte-like cells which secrete glial cell line-derived neurotrophic factor (GDNF), a widely-distributed protein that has been shown to prevent cell death in Parkinson’s disease and ALS. The reprogrammed cells originated in the patient’s own bone marrow, so can be reintroduced without triggering an immune response. The Food and Drug Administration has approved the stem cell regimen for “fast track” trials in ALS in the United States, and a library of pluripotent adult stem cells from ALS patients has been developed at Johns Hopkins University for further studies [17].
CONTRIBUTIONS OF BIOTECHNOLOGY
The extraction, redifferentiation and reinfusion of stem cells so as to deliver neuroproduction to diseased motor neurons is an illustration of the potential benefits that biotechnology can offer a serious neurological disorder historically resistant to medical therapy. The long career of Stephen Hawking is another illustration of the assistance that technology can give to continued high-level functioning in neurological disorders. A series of computer-based voice simulations have been developed for his use over the years, initially controlled by his functioning hand but later using a dwindling number of functioning facial muscles. As these have deteriorated, infrared glasses have been used to detect minimal cheek muscle contractions to activate the speech synthesizer. A backup system has been developed for switch activation by facial expression, making use of the extraocular muscle preservation that is characteristic of the disease, and he is currently working on direct brain activation of communication devices using a single-channel device for acquisition of the electroencephalogram (EEG) signal long used for neurologic diagnosis and subjecting his EEG to frequency analysis, the output of which can be used as a trigger for assistive equipment [18].
REFERENCES
1. Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011). Amyotrophic lateral sclerosis. Lancet, 377(9769): 42-55.
2. Turner MR, Swash M, Ebers GC (2010). Lockhart Clarke’s contribution to the description of amyotrophic lateral sclerosis. Brain, 133(11): 3470-3479.
3. Hardiman O, van den Berg LH, Kiernan MC (2011). Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol, 7(11): 639-649.
4. Brooks BR, Miller RG, Swash M, Munsat TL (2000). El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler, 1(5): 293-299.
5. Eisen A (2009). Amyotrophic lateral sclerosis: A 40-year personal perspective. J Clin Neurosci, 16(4): 505-512.
6. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz M (2010). Clinical significance of the change of decline in ALSFRS-R. Amyotroph Lateral Scler, 11(1-2): 178-180.
7. Turner MR, Parton MJ, Shaw CE, Leigh PN, Al-Chalabi A (2003). Prolonged survival in motor neuron disease: a descriptive study of the King’s database, 1990-2002. J Neurol Neurosurg Psychiat, 74(7): 995-995.
8. Stephen Hawking serves as a role model for ALS patients. CNN, April 21, 2009.
9. Sontheimer H (2015). Diseases of the Nervous System. Waltham, MA, Academic Press, pp. 165-172.
10. Battistini S, Ricci C, Lotti EM, Benigni M, Gagliardi S, Zucco R, Bondavalli M, Marcello N, Ceroni M, Cereda C (2010). Severe familial ALS with a novel exon 4 mutation (L106F) in the SOD1 gene. J Neurol Sci, 293(1): 112-115.
11. DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R (2011). Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9-linked FTD and ALS. Neuron, 72(2): 245-256.
12. Deng H-X, Chen W, Hong S-T, Boykott K, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, Jiang H, Hirano M, Rampersaud E, Jansen GH, Dankervoort S, Bigio H, Brooks BR, Ajroud K, Sufit RL, Haines JL, Mugnaini E, Pericak-Vance MA, Siddique T (2011). Mutations in UBQLN2 gene cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature, 477: 211-215.
13. Al-Chalabi A, Leigh PN ( 2000). Recent advances in amyotrophic lateral sclerosis. Current Opinion in Neurology, 13(4): 397–405.
14. Manuel M, Heckman CJ ( 2011). Stronger is not always better: could a bodybuilding dietary supplement lead to ALS? Exp Neurol, 228(1): 1-5.
15. Rodgers KJ ( 2014). Non-protein amino acids and neurodegeneration: The enemy within. Exp Neurol, 252(3): 192-196.
16. Miller RG, Mitchell JD, Lyon M, Moore DH (2007). Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database System Rev, 1: CD001447.
17. Li Y, Balasubramanian U, Cohen D, Zhang P-W, Mosmiller E, Sattler R, Maragakis NJ, Rothstein JD (2015). A Comprehensive Library of Familial Human Amyotrophic Lateral Sclerosis Induced Pluripotent Stem Cells. PLOS One, 10(3): e011826. doi 10.1371/journal.pone.0118266.
Related images from the web
Stephen Hawking
![[Image: 224239main_hawkingImage-13.jpg]](http://www.nasa.gov/images/content/224239main_hawkingImage-13.jpg)
![[Image: ei_2709.jpg]](http://www.uchospitals.edu/images/gs/ei_2709.jpg)
Source: htttp://www.uchospitals.edu/images/gs/ei_2709.jpg
by Utkarsh kumar at 05-05-2015, 11:03 PM
8 comments
Biotechnology and life science related summer internship programs or any industrial training opportunities for Indian students. Help others if you know about any such training.
ALSO CHECK THIS OUT: Indian Universities/Institutes Offering "Selection Based" Summer Internship Annually
-------------------------------------------------------------------------------------------
Any Indian students seeking Internship or Industrial Training - Please use this thread only.
Also if you know about any any such openings from reputed institutes , please post here as well.
Do not use this thread for any commercial purpose.
Thank you
- Forums Admin
( Help us in keeping the forums clean)
-------------------------------------------------------------------------------------------
Sir I am B.tech 3rd year student seeking for industrial training in biotech companies from Mumbai or Goa this summer.kindly help me
- Utkarsh
ALSO CHECK THIS OUT: Indian Universities/Institutes Offering "Selection Based" Summer Internship Annually
-------------------------------------------------------------------------------------------
Any Indian students seeking Internship or Industrial Training - Please use this thread only.
Also if you know about any any such openings from reputed institutes , please post here as well.
Do not use this thread for any commercial purpose.
Thank you
- Forums Admin
( Help us in keeping the forums clean)
-------------------------------------------------------------------------------------------
Sir I am B.tech 3rd year student seeking for industrial training in biotech companies from Mumbai or Goa this summer.kindly help me
- Utkarsh
by tansel.ozyer at 05-04-2015, 02:38 PM
0 comments
International Symposium on Network Enabled Health Informatics, Biomedicine and Bioinformatics (HI-BI-BI 2015)
Paris, France, August 27-28, 2015
http://hi-bi-bi.cpsc.ucalgary.ca/2015/
The advancement in technology and computational science influenced a wide range of fields, including research in clinical leading to health informatics as emerging vital research area which is attracting more attention in academia and industry. Health informatics combines computational science and the clinical world for better treatment of patients; it deals with resources, devices, and methodologies required for optimizing the acquisition, storage, maintenance, retrieval, and use of healthcare information and resources for effective diagnosis and treatment of patients. The target of this research track is to bring together professionals, researchers and practitioners in the area of health informatics to present, discuss, share the latest finding in the field, and exchange ideas that address real-world problems with real-world solutions..
All accepted papers will be published in the proceedings which will be included in the digital libraries of both sponsors: ACM and IEEE Computer Society.
IMPORTANT DATES:
------------------------
Full Paper Submission Deadline: May 17, 2015
Notification of acceptance: June 19, 2015
International Symposium on Network Enabled Health Informatics, Biomedicine and Bioinformatics (HI-BI-BI 2015):
The target of this research track is to bring together professionals, researchers and practitioners in the area of health informatics to present, discuss, share the latest finding in the field, and exchange ideas that address real-world problems with real-world solutions..
Camera-ready papers due: July 3, 2015
Conference events: August 26-27, 2015
--
Assoc. Prof. Tansel Özyer
Department of Computer Engineering,
TOBB Economics and Technology University,
Sogutozu Cad No 43 Sogutozu Ankara Turkey
Home Page: http://bil.etu.edu.tr/ozyer
Paris, France, August 27-28, 2015
http://hi-bi-bi.cpsc.ucalgary.ca/2015/
The advancement in technology and computational science influenced a wide range of fields, including research in clinical leading to health informatics as emerging vital research area which is attracting more attention in academia and industry. Health informatics combines computational science and the clinical world for better treatment of patients; it deals with resources, devices, and methodologies required for optimizing the acquisition, storage, maintenance, retrieval, and use of healthcare information and resources for effective diagnosis and treatment of patients. The target of this research track is to bring together professionals, researchers and practitioners in the area of health informatics to present, discuss, share the latest finding in the field, and exchange ideas that address real-world problems with real-world solutions..
All accepted papers will be published in the proceedings which will be included in the digital libraries of both sponsors: ACM and IEEE Computer Society.
IMPORTANT DATES:
------------------------
Full Paper Submission Deadline: May 17, 2015
Notification of acceptance: June 19, 2015
International Symposium on Network Enabled Health Informatics, Biomedicine and Bioinformatics (HI-BI-BI 2015):
The target of this research track is to bring together professionals, researchers and practitioners in the area of health informatics to present, discuss, share the latest finding in the field, and exchange ideas that address real-world problems with real-world solutions..
Camera-ready papers due: July 3, 2015
Conference events: August 26-27, 2015
--
Assoc. Prof. Tansel Özyer
Department of Computer Engineering,
TOBB Economics and Technology University,
Sogutozu Cad No 43 Sogutozu Ankara Turkey
Home Page: http://bil.etu.edu.tr/ozyer
by BioWritEditors at 05-03-2015, 05:47 PM
0 comments
Dear Participants,
The results of Biowrit (April 2015) are out and here is the list of winners. Most entries were good and results were equally close.
>> US Dollars:
(i) Please email us your Paypal Email Address. (We prefer this)
** or **
(ii) Amazon.com (USA) gift voucher.
>> Indian Rupees:
(i) Please send us your Prepaid Mobile Phone number and Service Provider and we will recharge your phone to the nearest talk time.
** or **
(ii) Flipkart India gift voucher.
Winners, please send us your email address (for Paypal/Flipkart) or your prepaid phone number (for mobile recharge), depending on your preference before 22th May 2015. We will finish the payment with 1-2 days of receiving your payment preference/details. No payments will be made after 25th May, so please send the details ASAP.
Our email address: BiotechnologyForums.com@gmail.com
Your email address and phone number will not be shared or published. If you want your photo to be published on the winners page (this page), please email that as well.
Rank-1 : Rs 2000/- ( $33 )
>> Bharat Manna - My Experience with GATE : The Gateway to IITs & NITs
Rank-2 : Rs 1500/- ( $25 )
>> ADAS - Screening and Interview at IIT Kanpur: My personal perspective
Rank-3 (Tie) : Rs 1000/- each ( $16 )
>> MannBhargavi - My Semester Abroad
>> Dr. Nitin Wahi - Making Career with CSIR-NET-JRF: Life Science
Rank-4 to 8 ; Rs 300/- ($5)
(In random order)
>> Dr. Nitin Wahi - No Knowledge without College
>> ADAS - Interview at IIT Kharagpur: My perspective
>> raj150194 - Metagenomics- exploring the microbes in their habitat
>> MannBhargavi - Clinical Trials in Drug Approval
>> shreyashivarshneya - GLA University : A place where I learned to become more self confident !
Entries which could not make it:
>> Ritu Yadav >> Clinical Trials In Drug Approval ( Article contents were copy-pasted from other sources, lacks originality)
>> Jimit90 >> Synergia - A web application ( Article was not fitting in the list of topics we had proposed for the contest)
1. Relevance and quality of content
2. Originality (Plagiarism level)
3. Quality of Title
4. Formatting of the article
5. Grammatical accuracy
6. References
7. Adherence to word limits
Winners were decided by an independent panel of 3 judges.
Author Photos: (Only those winners who sent their photos to us)
Bharat Manna

Dr. Nitin Wahi

Shreyashi Varshneya

Disclaimer: The decision of our review committee is final and no requests to review the entries will be further entertained.
The results of Biowrit (April 2015) are out and here is the list of winners. Most entries were good and results were equally close.
Participants who want to receive the amount in:
>> US Dollars:
(i) Please email us your Paypal Email Address. (We prefer this)
** or **
(ii) Amazon.com (USA) gift voucher.
>> Indian Rupees:
(i) Please send us your Prepaid Mobile Phone number and Service Provider and we will recharge your phone to the nearest talk time.
** or **
(ii) Flipkart India gift voucher.
Action required from winners
Winners, please send us your email address (for Paypal/Flipkart) or your prepaid phone number (for mobile recharge), depending on your preference before 22th May 2015. We will finish the payment with 1-2 days of receiving your payment preference/details. No payments will be made after 25th May, so please send the details ASAP.
Our email address: BiotechnologyForums.com@gmail.com
Your email address and phone number will not be shared or published. If you want your photo to be published on the winners page (this page), please email that as well.
Winners list !!
Rank-1 : Rs 2000/- ( $33 )
>> Bharat Manna - My Experience with GATE : The Gateway to IITs & NITs
Rank-2 : Rs 1500/- ( $25 )
>> ADAS - Screening and Interview at IIT Kanpur: My personal perspective
Rank-3 (Tie) : Rs 1000/- each ( $16 )
>> MannBhargavi - My Semester Abroad
>> Dr. Nitin Wahi - Making Career with CSIR-NET-JRF: Life Science
Rank-4 to 8 ; Rs 300/- ($5)
(In random order)
>> Dr. Nitin Wahi - No Knowledge without College
>> ADAS - Interview at IIT Kharagpur: My perspective
>> raj150194 - Metagenomics- exploring the microbes in their habitat
>> MannBhargavi - Clinical Trials in Drug Approval
>> shreyashivarshneya - GLA University : A place where I learned to become more self confident !
Entries which could not make it:
>> Ritu Yadav >> Clinical Trials In Drug Approval ( Article contents were copy-pasted from other sources, lacks originality)
>> Jimit90 >> Synergia - A web application ( Article was not fitting in the list of topics we had proposed for the contest)
Criteria of Judging
1. Relevance and quality of content
2. Originality (Plagiarism level)
3. Quality of Title
4. Formatting of the article
5. Grammatical accuracy
6. References
7. Adherence to word limits
Winners were decided by an independent panel of 3 judges.
Congratulations Everyone !!
Author Photos: (Only those winners who sent their photos to us)
Bharat Manna
Dr. Nitin Wahi
Shreyashi Varshneya
Disclaimer: The decision of our review committee is final and no requests to review the entries will be further entertained.
by abhishekiyer at 05-03-2015, 03:59 PM
2 comments
Hello,
I would like some advice. I had given GATE 2015 earlier this year and have obtained a GATE Score of 685 and a rank of 71. Does every IIT conduct an interview for biotechnology? What do they ask in the interview? Which IIT do i have a chance of getting into? I have been called for interview at IIsc for a direct phD. Do i go for a direct phD if i clear the interview? Or do i stick with M.Tech and then go for phD?? I also qualified JAM 2015 with a rank of 125. Do i have a chance for MSc at IIT Bombay through JAM??
I would like some advice. I had given GATE 2015 earlier this year and have obtained a GATE Score of 685 and a rank of 71. Does every IIT conduct an interview for biotechnology? What do they ask in the interview? Which IIT do i have a chance of getting into? I have been called for interview at IIsc for a direct phD. Do i go for a direct phD if i clear the interview? Or do i stick with M.Tech and then go for phD?? I also qualified JAM 2015 with a rank of 125. Do i have a chance for MSc at IIT Bombay through JAM??

![[Image: james-parkinson.jpg]](http://www.allaboutparkinsons.com/james-parkinson.jpg)
![[Image: acne.gif]](http://oliviajenkins.com.au/wp-content/uploads/2015/01/acne.gif)
![[Image: 1048885-1072716-2320tn.jpg]](http://img.medscape.com/pi/emed/ckb/dermatology/1048885-1072716-2320tn.jpg)
![[Image: als_graphic1.jpg]](http://www.al.com/images/specialreport/huntsvilletimes/als_graphic1.jpg)