Contact:
sales@biotechnologyforums.com to feature here
sales@biotechnologyforums.com to feature here
by NoahMachuki at 04-28-2013, 06:52 PM
2 comments
Plant tissue culture is a biotechnology application that utilizes a commercial nutrient culture medium to produce clones of plant cells, tissues, seeds or organs under sterile conditions. Plant tissue culture took off in 1962 when Murashige and Skoog discovered the first reliable artificial medium. Thereafter, major discoveries took place in the advancement of tissue culture;
a.) 1992 - Habertlandt attempted to grow leaf palisade cells from different plants. However, these cells did not divide.
b.) 1934 - White established the need to supplement the medium with additives. He grew meristematic cells of tomato on a medium supplemented with yeast extracts, Vit B (thiamine, pyridoxine, and nicotinic acid), salts and sucrose.
c.) 1953 - Skoog and Miller discovered kinetin. This is a cytokine that plays a central role in organogenesis.
d.) 1959 - Orchids were discovered by Morel. These orchids were set free from viral diseases by Dahlias in 1960
e.) 1960's - Murashige and Skoog cloned plants invitro after discovering and publishing a recipe for Murashige and Skoog (M&S) medium.
f.) 1970's and 80’s marked the genesis of genetic engineering in tissue culture.
There are two main facts that scientists rely upon in plant tissue culture. Plants are totipotent and have ability to produce callus. Totipotency is the ability of a cell to develop into a whole plant or a plant organ when subjected to the right conditions. However, not all plant cells are totipotent. A callus is a mass of actively dividing undifferentiated cells produced by a plant tissue explant- an isolated portion of a plant that is used to initiate a culture (an inoculum)
Plants are sessile in nature and have long life span thus have developed plasticity. Plasticity is the ability to survive and adapt predation and extreme conditions than animals. They alter their metabolic processes and growth to suit the environment.
In plant tissue culture, ability of plants to initiate cell division from most of the tissues of a plant and the developmental responses to stimuli has been of a major interest. This plasticity allows one type organ of a plant to be produced from another hence regeneration of a whole plant when exposed to correct stimuli.
Plant tissue culture requirement
In order to initiate plant tissue culture, one needs; explants, suitable culture/growth media, aseptic conditions to curb growth of microorganisms, water, growth regulators (auxins and cytokinins), and frequent sub culturing to avoid accumulation of waste metabolite and enhance nutrition.
Culture medium
Plant tissue culture medium that is meant for cultivation of plant cells in vitro should contain mineral ions or essential elements in form of a mixture of complex salts, a source of carbon which is usually sucrose and organic supplements that supply vitamins and amino acids.
Organic supplements
Organic supplements supply vitamins and amino acids. The two main vitamins essential for in vitro tissue culture are myoinositol and thiamine while the most important amino acid is glycine.
Carbon source
Sucrose is the preferred carbon source essential in a culture medium. It is easily available, cheap, easily assimilated and stable.
Essential elements/nutrients
Essential elements are classified as macronutrients, micronutrients and an iron source. A combination of these elements is necessary for tissue culture.
Macronutrients are those elements that are supplied in large amounts for plant growth and development. They include; magnesium, sulphur, calcium, nitrogen, potassium, phosphorus and carbon which supplied separately. All these elements comprise more than 0.1% of plants’ dry weight. Nitrogen is mainly supplied as nitrate ions or ammonium ions. However, high concentrations of ammonium ions lead to acidification of the medium and increase vitrification.
Microelements are those elements that are needed in trace amounts in tissue culture media. However, they have diverse functions in plant growth and development. These elements include; iron, molybdenum, Manganese, iodine, cobalt, copper, boron and zinc.
Role of growth regulators in tissue culture
Due to the totipotency and plasticity of plant cells, certain manipulations to culture media are essential to determine certain developmental pathways of a plant cell. Plant hormones and their respective synthetic analogues are used as plant growth regulators. These growth regulators include;
Auxins- promote cell division and growth in explants. They support callus induction hence growth. However, high levels of auxins suppress organized growth promoting growth of meristem-like cells. The mostly used type of auxin for tissue culture is called 2, 4-Dichlorophenoxyacetic acid (2, 4-D).
Cytokinins- these are purine derivatives that support cell division. The two main types of cytokinins used in tissue culture are benzylaminopurine (BAP) and kinetine.
Gibberellins - these are naturally occurring compounds that are used in regulating plant cell elongation. GA3 is the most commonly used type of gibberellin in this technique.
Abscisic acid (ABA) - inhibits cell division in plants. It is mostly used in somatic embryogenesis to promote specific developmental pathways.
Auxins and cytokinins, as growth regulators, have basic roles to play in plant tissue culture. Often, they are used together but with different concentration rations which subsequently determine the type of culture regenerated. A high cytokinin to auxin ratio supports formation of shoots, whereas a high auxin to cytokinin ratio favors formation of roots. A balanced ratio favors production of callus.
Micropropagation procedure
1. Selection of an explant from a ‘mother plant’ that is healthy and vigorous. Usually, apical buds are preferred as explants but any other tissue can be used.
2. Establishment of this explant in a plant culture medium. A medium supports growth and cell division. Depending on the plant requirement, different types of media are used for specific types of plants.
3. Multiplication. In this stage, the explants give rise to a callus.
4. Differentiation and
5. Organogenesis
Culture types
Cultures are produced from ‘explants’. There are different types of cultures produced from explants depending on the conditions availed. They include;
Callus
This is an unorganized, growing and actively dividing mass of cells produced when both auxins and cytokinins are present in a culture medium, a procedure carried out in the dark to discourage differentiation. During formation of callus, there is morphological and metabolic dedifferentiation. Dedifferentiation results into inability of these cultures to photosynthesize hence attain a different metabolic profile from the ‘mother plant’. This feature precipitates addition of other culture components.
Manipulation of auxin to cytokinin ratios dictates root, shoot and somatic embryo development from which plants are produced. Callus cultures are classified as either compact or friable. Callus formation plays a central role in plant biotechnology.
Cell- suspension cultures
These cultures are produced from friable callus placed in a liquid medium and agitated. This releases single cells into the medium which under correct conditions, grow and divide to produce cell-suspension cultures. These cells are maintained as batch cultures in flasks.
Protoplasts
These are plant cells without cell walls. Removal of cell walls can be done either mechanically or by use of enzymes. The former method results in poor quality yields while the latter yields high and pure cells. The liquid medium used is not agitated to avoid damaging the protoplasts. However, the medium is put maintained under high osmotic pressure and shallow to allow aeration. Organogenesis or somatic embryogenesis can be used to produce whole plants on solid media. Many transformations are done through this method.
Embryo culture
Embryos are used to produce either a callus culture or a somatic embryo. An immature embryo from an embryogenic callus is the most recommended for regeneration of monocot plants.
Other culture types include; microspore culture, root cultures and shoot tip and meristem cultures. These cultures give rise to plant regeneration.
a.) 1992 - Habertlandt attempted to grow leaf palisade cells from different plants. However, these cells did not divide.
b.) 1934 - White established the need to supplement the medium with additives. He grew meristematic cells of tomato on a medium supplemented with yeast extracts, Vit B (thiamine, pyridoxine, and nicotinic acid), salts and sucrose.
c.) 1953 - Skoog and Miller discovered kinetin. This is a cytokine that plays a central role in organogenesis.
d.) 1959 - Orchids were discovered by Morel. These orchids were set free from viral diseases by Dahlias in 1960
e.) 1960's - Murashige and Skoog cloned plants invitro after discovering and publishing a recipe for Murashige and Skoog (M&S) medium.
f.) 1970's and 80’s marked the genesis of genetic engineering in tissue culture.
There are two main facts that scientists rely upon in plant tissue culture. Plants are totipotent and have ability to produce callus. Totipotency is the ability of a cell to develop into a whole plant or a plant organ when subjected to the right conditions. However, not all plant cells are totipotent. A callus is a mass of actively dividing undifferentiated cells produced by a plant tissue explant- an isolated portion of a plant that is used to initiate a culture (an inoculum)
Plants are sessile in nature and have long life span thus have developed plasticity. Plasticity is the ability to survive and adapt predation and extreme conditions than animals. They alter their metabolic processes and growth to suit the environment.
In plant tissue culture, ability of plants to initiate cell division from most of the tissues of a plant and the developmental responses to stimuli has been of a major interest. This plasticity allows one type organ of a plant to be produced from another hence regeneration of a whole plant when exposed to correct stimuli.
Plant tissue culture requirement
In order to initiate plant tissue culture, one needs; explants, suitable culture/growth media, aseptic conditions to curb growth of microorganisms, water, growth regulators (auxins and cytokinins), and frequent sub culturing to avoid accumulation of waste metabolite and enhance nutrition.
Culture medium
Plant tissue culture medium that is meant for cultivation of plant cells in vitro should contain mineral ions or essential elements in form of a mixture of complex salts, a source of carbon which is usually sucrose and organic supplements that supply vitamins and amino acids.
Organic supplements
Organic supplements supply vitamins and amino acids. The two main vitamins essential for in vitro tissue culture are myoinositol and thiamine while the most important amino acid is glycine.
Carbon source
Sucrose is the preferred carbon source essential in a culture medium. It is easily available, cheap, easily assimilated and stable.
Essential elements/nutrients
Essential elements are classified as macronutrients, micronutrients and an iron source. A combination of these elements is necessary for tissue culture.
Macronutrients are those elements that are supplied in large amounts for plant growth and development. They include; magnesium, sulphur, calcium, nitrogen, potassium, phosphorus and carbon which supplied separately. All these elements comprise more than 0.1% of plants’ dry weight. Nitrogen is mainly supplied as nitrate ions or ammonium ions. However, high concentrations of ammonium ions lead to acidification of the medium and increase vitrification.
Microelements are those elements that are needed in trace amounts in tissue culture media. However, they have diverse functions in plant growth and development. These elements include; iron, molybdenum, Manganese, iodine, cobalt, copper, boron and zinc.
Role of growth regulators in tissue culture
Due to the totipotency and plasticity of plant cells, certain manipulations to culture media are essential to determine certain developmental pathways of a plant cell. Plant hormones and their respective synthetic analogues are used as plant growth regulators. These growth regulators include;
Auxins- promote cell division and growth in explants. They support callus induction hence growth. However, high levels of auxins suppress organized growth promoting growth of meristem-like cells. The mostly used type of auxin for tissue culture is called 2, 4-Dichlorophenoxyacetic acid (2, 4-D).
Cytokinins- these are purine derivatives that support cell division. The two main types of cytokinins used in tissue culture are benzylaminopurine (BAP) and kinetine.
Gibberellins - these are naturally occurring compounds that are used in regulating plant cell elongation. GA3 is the most commonly used type of gibberellin in this technique.
Abscisic acid (ABA) - inhibits cell division in plants. It is mostly used in somatic embryogenesis to promote specific developmental pathways.
Auxins and cytokinins, as growth regulators, have basic roles to play in plant tissue culture. Often, they are used together but with different concentration rations which subsequently determine the type of culture regenerated. A high cytokinin to auxin ratio supports formation of shoots, whereas a high auxin to cytokinin ratio favors formation of roots. A balanced ratio favors production of callus.
Micropropagation procedure
1. Selection of an explant from a ‘mother plant’ that is healthy and vigorous. Usually, apical buds are preferred as explants but any other tissue can be used.
2. Establishment of this explant in a plant culture medium. A medium supports growth and cell division. Depending on the plant requirement, different types of media are used for specific types of plants.
3. Multiplication. In this stage, the explants give rise to a callus.
4. Differentiation and
5. Organogenesis
Culture types
Cultures are produced from ‘explants’. There are different types of cultures produced from explants depending on the conditions availed. They include;
Callus
This is an unorganized, growing and actively dividing mass of cells produced when both auxins and cytokinins are present in a culture medium, a procedure carried out in the dark to discourage differentiation. During formation of callus, there is morphological and metabolic dedifferentiation. Dedifferentiation results into inability of these cultures to photosynthesize hence attain a different metabolic profile from the ‘mother plant’. This feature precipitates addition of other culture components.
Manipulation of auxin to cytokinin ratios dictates root, shoot and somatic embryo development from which plants are produced. Callus cultures are classified as either compact or friable. Callus formation plays a central role in plant biotechnology.
Cell- suspension cultures
These cultures are produced from friable callus placed in a liquid medium and agitated. This releases single cells into the medium which under correct conditions, grow and divide to produce cell-suspension cultures. These cells are maintained as batch cultures in flasks.
Protoplasts
These are plant cells without cell walls. Removal of cell walls can be done either mechanically or by use of enzymes. The former method results in poor quality yields while the latter yields high and pure cells. The liquid medium used is not agitated to avoid damaging the protoplasts. However, the medium is put maintained under high osmotic pressure and shallow to allow aeration. Organogenesis or somatic embryogenesis can be used to produce whole plants on solid media. Many transformations are done through this method.
Embryo culture
Embryos are used to produce either a callus culture or a somatic embryo. An immature embryo from an embryogenic callus is the most recommended for regeneration of monocot plants.
Other culture types include; microspore culture, root cultures and shoot tip and meristem cultures. These cultures give rise to plant regeneration.
by bridgettpayseur at 04-28-2013, 07:40 AM
1 comments
It’s a classic story line, straight from comic books. An ordinary, everyday person is exposed to radiation, thereby gaining super powers. This irradiated super hero is then able to go out and stop criminals, protecting the city. While the story is familiar, the players have changed. Recently, researchers have found that a strain of Listeria monocytogenes bacteria, the new super hero, can deliver radiation to pancreatic cancer metastasis, killing the tumor cells and saving the world from the evil cancer.
Pancreatic cancer is an extremely lethal form of cancer, with a very low 5 year survival rate and poor prognosis for patients. Metastasis of the primary pancreatic cancer tumor, which is when tumor cells move to and colonize other parts of the body, is one of many reasons why pancreatic cancer is so lethal. While removal of the pancreatic tumor may help slow down the damaged caused by the cancer, metastatic tumors can be more difficult to find, may not develop until after the primary tumor has been removed, and may not be as susceptible to standard treatments as primary tumor cells.
The use of microbes to fight cancer is not a new field of research. Many researchers have used bacteria to either directly kill tumor cells, or as a vector to deliver vaccines or medications. In fact, an immune factor known as Tumor Necrosis Factor was first discovered over one hundred years ago by a practitioner who injected cancer patients with live bacteria. The live bacteria helped incite an immune response that was effective against the tumors. Unfortunately, many of the patients succumbed to the bacterial infection and the extremely strong immune response that followed. In the past hundred years, research into this type of anticancer therapeutic has come a long way. Bacteria and viruses can more easily target and kill damaged cells, such as a cancer cells. By utilizing attenuated strains of bacteria or viruses, which are not as pathogenic as normal strains, scientists can target defective cells while leaving healthy cells unharmed by the microbe. In addition, bacteria and virus expressing tumor cell antigens have been used to help train the immune system to fight cancer cells.
Previously, an attenuated strain of L. monocytogenes was able to selectively kill breast cancer cells, without harming normal cells. This inspired researchers at the Albert Einstein College of Medicine to test whether the attenuated Listeria could be effective against metastatic pancreatic cancer cells. The researchers believed that the Listeria bacteria could be used either to directly kill the cancer cells, or to target the cells and bring medicine or radiation to the metastatic tumors. A study performed in a mouse model of pancreatic cancer indeed showed that the attenuated L. monocytogenes could effectively target metastatic tumor cells.
The researchers found that the attenuated L. monocytogenes replicated strongly in metastatic pancreatic cancer cells. The bacteria replicated less efficiently in primary tumor cells, and very poorly in normal cells. This suggested that the bacteria could be used to specifically target metastases, which are normally very difficult to find and treat. The researchers loaded the Listeria bacteria with a radioactive compound to deliver to the metastatic cancer cells. The metastatic pancreatic cancer tumors in mice treated with this combination therapy of bacterial killing and radiation therapy resulted in tumors shrinking 90% compared to tumors in mice treated with saline injections. The effects of killing by the attenuated L. monocytogenes bacteria was significantly enhanced by the radiation delivered to the tumor cells.
While the researcher suggests that the bacteria can be used to target and kill metastatic cancer cells, the results are not as straightforward as they seem. The radioactive L. monocytogenes bacteria were not given to the mice after the formation of metastasis. Rather, the treatment was provided while the metastases were being formed. Even though the research does not demonstrate that the treatment can destroy metastatic tumors that have already become established, it is potentially usable as a preventative treatment. Preventing metastasis of tumors from many types of cancer would be extremely beneficial. The L. monocytogenes treatment could also potentially be used therapeutically after a patient has completed preliminary anticancer chemotherapy, as a preventative measure against future metastases to prevent recurrence of the cancer.
References:
http://www.the-scientist.com/?articles.v...-Bacteria/
Pancreatic cancer is an extremely lethal form of cancer, with a very low 5 year survival rate and poor prognosis for patients. Metastasis of the primary pancreatic cancer tumor, which is when tumor cells move to and colonize other parts of the body, is one of many reasons why pancreatic cancer is so lethal. While removal of the pancreatic tumor may help slow down the damaged caused by the cancer, metastatic tumors can be more difficult to find, may not develop until after the primary tumor has been removed, and may not be as susceptible to standard treatments as primary tumor cells.
The use of microbes to fight cancer is not a new field of research. Many researchers have used bacteria to either directly kill tumor cells, or as a vector to deliver vaccines or medications. In fact, an immune factor known as Tumor Necrosis Factor was first discovered over one hundred years ago by a practitioner who injected cancer patients with live bacteria. The live bacteria helped incite an immune response that was effective against the tumors. Unfortunately, many of the patients succumbed to the bacterial infection and the extremely strong immune response that followed. In the past hundred years, research into this type of anticancer therapeutic has come a long way. Bacteria and viruses can more easily target and kill damaged cells, such as a cancer cells. By utilizing attenuated strains of bacteria or viruses, which are not as pathogenic as normal strains, scientists can target defective cells while leaving healthy cells unharmed by the microbe. In addition, bacteria and virus expressing tumor cell antigens have been used to help train the immune system to fight cancer cells.
Previously, an attenuated strain of L. monocytogenes was able to selectively kill breast cancer cells, without harming normal cells. This inspired researchers at the Albert Einstein College of Medicine to test whether the attenuated Listeria could be effective against metastatic pancreatic cancer cells. The researchers believed that the Listeria bacteria could be used either to directly kill the cancer cells, or to target the cells and bring medicine or radiation to the metastatic tumors. A study performed in a mouse model of pancreatic cancer indeed showed that the attenuated L. monocytogenes could effectively target metastatic tumor cells.
The researchers found that the attenuated L. monocytogenes replicated strongly in metastatic pancreatic cancer cells. The bacteria replicated less efficiently in primary tumor cells, and very poorly in normal cells. This suggested that the bacteria could be used to specifically target metastases, which are normally very difficult to find and treat. The researchers loaded the Listeria bacteria with a radioactive compound to deliver to the metastatic cancer cells. The metastatic pancreatic cancer tumors in mice treated with this combination therapy of bacterial killing and radiation therapy resulted in tumors shrinking 90% compared to tumors in mice treated with saline injections. The effects of killing by the attenuated L. monocytogenes bacteria was significantly enhanced by the radiation delivered to the tumor cells.
While the researcher suggests that the bacteria can be used to target and kill metastatic cancer cells, the results are not as straightforward as they seem. The radioactive L. monocytogenes bacteria were not given to the mice after the formation of metastasis. Rather, the treatment was provided while the metastases were being formed. Even though the research does not demonstrate that the treatment can destroy metastatic tumors that have already become established, it is potentially usable as a preventative treatment. Preventing metastasis of tumors from many types of cancer would be extremely beneficial. The L. monocytogenes treatment could also potentially be used therapeutically after a patient has completed preliminary anticancer chemotherapy, as a preventative measure against future metastases to prevent recurrence of the cancer.
References:
http://www.the-scientist.com/?articles.v...-Bacteria/
by SunilNagpal at 04-27-2013, 09:15 PM
4 comments
Since the day, Ashanti Desilva, a four year old little girl from United States, suffering from ADA SCID (an immune deficiency disease) was operated by the first ever gene therapy attempt in the medical history in 1990, a lot has happened in the field of Gene Therapy. Speculations were always rife about the fatal consequences of Gene Therapy (alteration of normal genetic make-up, physiological rejection etc), but they could never out-weigh the foreseen advantages of permanent cure of diseases as deadly as Cancer.
Modes of In-vivo Targeted Gene Delivery
The in-vivo procedure can utilize two modes of delivering the transgene to the target site:
a. Viral Mode
b. Non-Viral Mode
Our physiology is an outcome of the housekeeping genes, or in better words, our normal physiology is an outcome of the normal functioning of the housekeeping genes expressing round the clock in our system (body). Whereas, the house keeping genes ensure the optimal functioning of metabolic pathways ranging from DNA synthesis to food digestion, there are another set of genes called oncogenes (cancer causing genes) whose dormancy is extremely crucial for the normal functioning of the system. In dormant (or inactive) state, these oncogenes are termed proto-oncogenes and are unable to exert their effect i.e uncontrolled cell division. And, any mutation in either the house keeping genes or proto-oncogenes, can severely harm the normal functioning of the physiological system, manifested in the form of diseases like Thalassemia, Sickle Cell Anemia and numerous forms of cancers etc. This is where gene therapy pronounces itself as the most efficient and lasting cure for such fatal diseases; because it’s always best to remove the cause than to treat the symptoms.
The basic philosophy of the gene therapy is to replace the mal-functioning/mutated gene with a normal gene. In words, it might seem as simple as swapping an old ball with a new one, but in practice gene swapping is the most ambitious treatment for any disease, whose chances of success are as low as tracing a needle in the haystack! A successful gene therapy has numerous check lists to follow, missing a single pre-requisite can mar the attempt and rather pose threat to the subject. One of the most important requirement in gene therapy is targeted delivery of the gene(s) to the genome of the cell(s), it’s intended to swap genes with. As in the case of ADA SCID of Ashanthi, one of the targeted approach is to transfer the required gene into the target cells by taking them out of the body and them conducting an in-vitro transfer to the cultured cells. This approach, called ex-vivo, requires extraction of the desired cell(s) from the body, a high class culturing facility for the cells and then a mechanism for regular injection of those cells in the body at the extracted location, making it inherently cumbrous. Other method, the in-vivo mode of gene delivery, involves direct injection of the transgene into the body through various routes, with an aim of site-specific delivery, integration and expression of the gene of interest. Now, the term direct injection shouldn't be misinterpreted, as the DNA/transgene cannot be injected as such in naked form. A naked DNA may be treated as an antigen and defense response may be initiated by the body to wipe the antigen. Apart from that, various nucleases present in the body fluids/cells may degrade the naked DNA much before its anticipated delivery to the target site.
Modes of In-vivo Targeted Gene Delivery
The in-vivo procedure can utilize two modes of delivering the transgene to the target site:
a. Viral Mode
b. Non-Viral Mode
Viral mode exploits the ability of viral vectors to integrate their DNA into the host genome, and replicating with the host thereafter. This is one of the most efficient mode of gene transfer in terms of the probability of successful gene integration and expression. But the concern over the use of Viral vectors in Humans has always been a roadblock in this regard.
Considering the limitation of viral vectors, numerous attempts have been made in developing an efficient non-viral mode of gene delivery. Use of gene gun, polyplexes and lipoplexes, are some of the conventionally tried methods to deliver genes into the cells. But considering the stringent requirement of the gene therapy, the rate of success with such physical methods is very low. It is equally probable that the gene carrying complex may reach at an unintended site and integrate the DNA in the normal cells causing adverse side effects. The obvious challenge in using these physical means of delivery lies in targeted delivery of the delivery complex. In order to achieve a site specific delivery, tagging of these complexes with some ligands complementary to the surface antigens of the targeted cells is the most common and efficient approach. Infact, ligand tagged nanoparticles have emerged as the complexes of choice for delivering the genes to the target site. For example, Tissue Factor (TF) expressed by injured cells of the body has become an address of choice for nanoparticle mediated drug and gene delivery to the injured tissue inside the body. EGFP-EGF1 is tagged on the nanoparticles which owing to it’s affinity towards TF directs the nanoparticles carrying the drug/gene payload to the injured site. The nature of nanoparticles under use in most cases is PLGA or poly(lactic-co-glycolic acid), which exhibits extraordinary biocompatibility and biodegradability. In a recent publication by Department of Surgery, Guangdong Provincial Stomatological Hospital, Southern Medical University, Guangzhou, People's Republic of China, use of Quantum Dots (QDs) as vectors for targeted survivin gene siRNA delivery was reported. Use of QDs enables real time probing of the successful gene delivery and it’s expression levels. For those who are unaware of the concept of QDs, these are tiny nano-particles with a size range of 2-10nm and are chemically selenides of cadmium or zinc. Their extra-ordinary small size enables unique electrical and optical properties, which can be studied in the form of photonic emissions.
Evidently, a lot of progress has taken place in the field of targeted gene delivery for an efficient gene therapy. New approaches like ligand coated Nanoparticles and use of Quantum Dots exhibit some of the big achievements in this field in a period as short as just 2 decades. With the widening scope of targeted delivery, diseases like advanced Cancer and even HIV have been reportedly cured in some instances. And, the scope will keep expanding it’s horizons with increasing knowledge of genetic behavior of diseases and newer means of delivering the medicinal gene to the diseased site.
Considering the limitation of viral vectors, numerous attempts have been made in developing an efficient non-viral mode of gene delivery. Use of gene gun, polyplexes and lipoplexes, are some of the conventionally tried methods to deliver genes into the cells. But considering the stringent requirement of the gene therapy, the rate of success with such physical methods is very low. It is equally probable that the gene carrying complex may reach at an unintended site and integrate the DNA in the normal cells causing adverse side effects. The obvious challenge in using these physical means of delivery lies in targeted delivery of the delivery complex. In order to achieve a site specific delivery, tagging of these complexes with some ligands complementary to the surface antigens of the targeted cells is the most common and efficient approach. Infact, ligand tagged nanoparticles have emerged as the complexes of choice for delivering the genes to the target site. For example, Tissue Factor (TF) expressed by injured cells of the body has become an address of choice for nanoparticle mediated drug and gene delivery to the injured tissue inside the body. EGFP-EGF1 is tagged on the nanoparticles which owing to it’s affinity towards TF directs the nanoparticles carrying the drug/gene payload to the injured site. The nature of nanoparticles under use in most cases is PLGA or poly(lactic-co-glycolic acid), which exhibits extraordinary biocompatibility and biodegradability. In a recent publication by Department of Surgery, Guangdong Provincial Stomatological Hospital, Southern Medical University, Guangzhou, People's Republic of China, use of Quantum Dots (QDs) as vectors for targeted survivin gene siRNA delivery was reported. Use of QDs enables real time probing of the successful gene delivery and it’s expression levels. For those who are unaware of the concept of QDs, these are tiny nano-particles with a size range of 2-10nm and are chemically selenides of cadmium or zinc. Their extra-ordinary small size enables unique electrical and optical properties, which can be studied in the form of photonic emissions.
Evidently, a lot of progress has taken place in the field of targeted gene delivery for an efficient gene therapy. New approaches like ligand coated Nanoparticles and use of Quantum Dots exhibit some of the big achievements in this field in a period as short as just 2 decades. With the widening scope of targeted delivery, diseases like advanced Cancer and even HIV have been reportedly cured in some instances. And, the scope will keep expanding it’s horizons with increasing knowledge of genetic behavior of diseases and newer means of delivering the medicinal gene to the diseased site.
by NoahMachuki at 04-27-2013, 08:00 PM
1 comments
Metabolism involves all the biochemical reactions that take place in the cells of organisms. Metabolism is divided into anabolism and catabolism. Catabolism is the breakdown of organic substances to produce energy while anabolism is the biosynthesis of complex organic materials with the expenditure of energy. Proteins, lipids and carbohydrates are the major substrates for catabolism. These biochemical pathways are very integral in the body functioning. There are different enzymes that are involved in these anabolic and catabolic and anabolic pathways. Any defectiveness in these enzymes in all these enzymatic reactions results into metabolic disorders. Metabolic diseases can either be classified as either inherited or acquired. Inherited disorders are also known as inborn errors of metabolism or congenital metabolic diseases.
Amino acids are building blocks of proteins which are made of carbon, hydrogen, oxygen and nitrogen. Biosynthesis or break down of these amino acids yield different products that are important in the body. Inborn errors of amino acid metabolism are as a result of accumulation of toxic metabolic products in the body system or as a result of inefficient breakdown of amino acids and proteins (Lehninger, 2008). There are different inborn errors of amino acid metabolism which include; Medium-chain-acyl-CoA dehydrogenase (MCAD), Maple syrup urine disease, Hereditary tyrosinemia, Arginosuccinic aciduria, Transient hyperammonemia of the newborn, and Ornithine transcarbamylase deficiency.
Tyrosinemia is an inborn error disorder that is as a result of ineffective breakdown of tyrosine. Tyrosine is a polar nonessential amino acid that is coded by UAC or UAU. It is caused by the deficiency of enzymes that break down the amino acid (Lehninger, 2008). Tyrosine catabolism is as shown in the schematic diagram below;
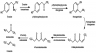
There are three types of tyrosinemia. They include tyrosinemia type I, type II and type III which are caused by deficiency of specific different enzymes. Tyrosinemia type I is a heritable condition that occurs in autosomal recessive pattern and is caused by the deficiency of the enzyme p-hydroxyphenylepyruvic acid oxidase and fumarylacetoacetate hydrolase. It is a very common disorder in Quebec in Canada. This disease is the most severe of the three tyrosinemia conditions. This enzyme takes part in the last stage of tyrosine catabolism where it catalyzes the breakdown of fumarylacetoacetate to fumarate.
Deficiency of fumarylacetoacetate hydrolase leads to increased amounts of fumarylacetoacetate in the hepatocytes which translates to inhibition of previous tyrosine breakdown processes. This leads to the accumulation of tyrosine in the body which causes dermatologic problems. This condition manifests itself in early months of life and affects the kidneys and the liver. It leads to conjugated hyperbilirubinemia, high frequency of Acute Flaccid Paralysis (AFP), liver cirrhosis, coagulation abnormalities hypoglycemia and fanconi syndrome as a result of kidney dysfunction (Annora G. et al). Often, symptoms include; vomiting, skin turns yellowish, jaundice, and diarrhea, slow rate of growth, regular nose bleeding and production of cabbage-like odor. When severe, it can affect the nervous system, cause liver cancer and kidney and liver failureCurrently, there are three known ways of treating tyrosinemia type I which are dietary treatment, seeking medication and lastly undertaking a liver transplant. People with tyrosinemia I have a specially prescribed diet that does not contain large amounts of tyrosine. For young children who are infected with the disease, they are fed with infant formula and natural foods. Furthermore, the health status, age and weight of the child should be carefully monitored to regulate and adjust the amount of diet that is provided.
Deficiency of Tyrosine aminotransferase which is encoded by TAT gene causes Type II tyrosinemia. This enzyme catalyzes the first reaction step in the catabolism of tyrosine.it is also inherited in an autosomal recessive way. This disorder, at early childhood causes photophobia, excessive tearing, retarded mental development, affects the skin and eyes, causes redness pain in the eyes, painful skin lesions and lastly reduces intellectual development. This type of disease is controlled by reducing intake of high and phenylalanine and tyrosine containing foods.
Type III tyrosinemia is not so rampart in societies. It is caused by a hydroxyphenylpyruvate dioxygenase gene encoded hydroxyphenylpyruvate dioxygenase enzyme which aids the reaction of conversion of 4-hydroxyphenylpyruvate to homogentisic acid (Storey K., 2005). It is caused by exaggerated levels of tyrosine in the diet in newborns. This condition is characterized by seizures, intermittent ataxia and menta retardation. It is recommended to take large amounts of Vitamin C and low small amounts of phenylalanine containing foods.
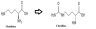
Ornithine transcarbamylase deficiency (OTCD) is an inherited condition that is also passed to the offspring when both parents are infected. This is a urea cycle genetic disease that is caused by mutating the OTC enzyme. Ornithine transcarbamylase enzyme takes part in the urea cycle by converting carbamylase phosphate and orthinine to citrulline. Urea cycle is responsible for getting rid of the excess toxic ammonia generated by nitrogen produced from the deamination processes in the amino acid catabolism in form of urine in the liver leading to detoxification.When there is deficiency of this enzyme, there is accumulation of ammonia in the system damaging the nervous system damaging the liver by enhancing neurological defects. This disorder often becomes evident et the early stages of life. Infants suffering from this disorder have uncontrolled body temperatures, are lethargic, poor breathing rates and coma. There is also limited intellectual development, skin lesions, liver damage, and growth of brittle hair.
The disorder is prevented and treated dietary through eating of foods that are low in protein amounts to reduce excess production of ammonia in the body.
Maple syrup urine disease (MSUD) is also another autosomal recessive disorder that affects amino acids that are branched. It is also called branched chain ketoaciduria. The branched chain amino acids include leucine, valine and isoleucine.
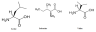
This disorder is caused by a deficiency of branched chain alpha-keto acid dehydrogenase complex-BCKDC (Annora G. et al). This deficiency leads to accumulation of these three amino acids in blood. Further, an increased level of toxic metabolites of these amino acids is also observed in urine through the sweet odor that they produce as they produce sotolone compound. During birth, infants look normal but with time, they can suffer from severe damages of the brain if medication or treatment is not sought earlier enough. This condition shows dehydration, seizures, ketoacidosis, coma, lethargy and hypoglycemia. Maple syrup urine is genetically classified or it can be through the sequence of its signs and symptoms. It could be divided into intermediate MSUD, Classic severe MSUD, Thiamine responsive MSUD, Intermittent MSUD and E3-Deficient MSUD. Classic severe is the most wide spread and severe form known so far that manifests itself in a newborn at birth. Since this disease is caused by amino acids, to avoid it, people are advised to eat a diet that is less concentrated in proteins.
Argininosuccinic aciduria is also inherited condition that leads to production of high amounts of argininosuccinic acid in urine and blood. It develops from the citric acid cycle where Argininosuccinate lyase (ASL)enzyme is used to breakdown argininosuccinate to arginine, fumarate and dicarboxylic acid.Any mutation of this enzyme results into a reduced enzyme activity.
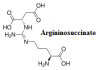
Argininosuccinate lyase is made of four monomers which are identical. For its catalysis, it has four active sites that help in the catalysis of argininosuccinate catabolism. Fumarate and arginine are formed from the breakdown of argininosuccinate using elimination method. This reaction step is the fourth one in a series of reaction in the urea cycle. At this stage fumarate enters the tricarboxylic acid cycle ( TCA) while arginine yields urea and orthinine. When this enzyme is mutated, it causes argininosuccinic aciduria which is also an autosomal recessive disorder which is not common.This leads to accumulation of compounds in the body which leads to hypothermia, vomiting, lethargy, muscle weakness, liver enlargement hyperventilation, mental retardation and hepatic fibrosis. The condition is managed through reduction of intake food with high protein contents.
Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency is an inherited autosomal disease which is as a result of amino acid catabolism. It actually prevents some fats from being converted to energy. This is mostly experienced during starvation and fasting. It is characterized by hypoglycemia or low blood sugar, lethargy or lack of energy and vomiting.
Amino acids are building blocks of proteins which are made of carbon, hydrogen, oxygen and nitrogen. Biosynthesis or break down of these amino acids yield different products that are important in the body. Inborn errors of amino acid metabolism are as a result of accumulation of toxic metabolic products in the body system or as a result of inefficient breakdown of amino acids and proteins (Lehninger, 2008). There are different inborn errors of amino acid metabolism which include; Medium-chain-acyl-CoA dehydrogenase (MCAD), Maple syrup urine disease, Hereditary tyrosinemia, Arginosuccinic aciduria, Transient hyperammonemia of the newborn, and Ornithine transcarbamylase deficiency.
Tyrosinemia is an inborn error disorder that is as a result of ineffective breakdown of tyrosine. Tyrosine is a polar nonessential amino acid that is coded by UAC or UAU. It is caused by the deficiency of enzymes that break down the amino acid (Lehninger, 2008). Tyrosine catabolism is as shown in the schematic diagram below;
There are three types of tyrosinemia. They include tyrosinemia type I, type II and type III which are caused by deficiency of specific different enzymes. Tyrosinemia type I is a heritable condition that occurs in autosomal recessive pattern and is caused by the deficiency of the enzyme p-hydroxyphenylepyruvic acid oxidase and fumarylacetoacetate hydrolase. It is a very common disorder in Quebec in Canada. This disease is the most severe of the three tyrosinemia conditions. This enzyme takes part in the last stage of tyrosine catabolism where it catalyzes the breakdown of fumarylacetoacetate to fumarate.
Deficiency of fumarylacetoacetate hydrolase leads to increased amounts of fumarylacetoacetate in the hepatocytes which translates to inhibition of previous tyrosine breakdown processes. This leads to the accumulation of tyrosine in the body which causes dermatologic problems. This condition manifests itself in early months of life and affects the kidneys and the liver. It leads to conjugated hyperbilirubinemia, high frequency of Acute Flaccid Paralysis (AFP), liver cirrhosis, coagulation abnormalities hypoglycemia and fanconi syndrome as a result of kidney dysfunction (Annora G. et al). Often, symptoms include; vomiting, skin turns yellowish, jaundice, and diarrhea, slow rate of growth, regular nose bleeding and production of cabbage-like odor. When severe, it can affect the nervous system, cause liver cancer and kidney and liver failureCurrently, there are three known ways of treating tyrosinemia type I which are dietary treatment, seeking medication and lastly undertaking a liver transplant. People with tyrosinemia I have a specially prescribed diet that does not contain large amounts of tyrosine. For young children who are infected with the disease, they are fed with infant formula and natural foods. Furthermore, the health status, age and weight of the child should be carefully monitored to regulate and adjust the amount of diet that is provided.
Deficiency of Tyrosine aminotransferase which is encoded by TAT gene causes Type II tyrosinemia. This enzyme catalyzes the first reaction step in the catabolism of tyrosine.it is also inherited in an autosomal recessive way. This disorder, at early childhood causes photophobia, excessive tearing, retarded mental development, affects the skin and eyes, causes redness pain in the eyes, painful skin lesions and lastly reduces intellectual development. This type of disease is controlled by reducing intake of high and phenylalanine and tyrosine containing foods.
Type III tyrosinemia is not so rampart in societies. It is caused by a hydroxyphenylpyruvate dioxygenase gene encoded hydroxyphenylpyruvate dioxygenase enzyme which aids the reaction of conversion of 4-hydroxyphenylpyruvate to homogentisic acid (Storey K., 2005). It is caused by exaggerated levels of tyrosine in the diet in newborns. This condition is characterized by seizures, intermittent ataxia and menta retardation. It is recommended to take large amounts of Vitamin C and low small amounts of phenylalanine containing foods.
Ornithine transcarbamylase deficiency (OTCD) is an inherited condition that is also passed to the offspring when both parents are infected. This is a urea cycle genetic disease that is caused by mutating the OTC enzyme. Ornithine transcarbamylase enzyme takes part in the urea cycle by converting carbamylase phosphate and orthinine to citrulline. Urea cycle is responsible for getting rid of the excess toxic ammonia generated by nitrogen produced from the deamination processes in the amino acid catabolism in form of urine in the liver leading to detoxification.When there is deficiency of this enzyme, there is accumulation of ammonia in the system damaging the nervous system damaging the liver by enhancing neurological defects. This disorder often becomes evident et the early stages of life. Infants suffering from this disorder have uncontrolled body temperatures, are lethargic, poor breathing rates and coma. There is also limited intellectual development, skin lesions, liver damage, and growth of brittle hair.
The disorder is prevented and treated dietary through eating of foods that are low in protein amounts to reduce excess production of ammonia in the body.
Maple syrup urine disease (MSUD) is also another autosomal recessive disorder that affects amino acids that are branched. It is also called branched chain ketoaciduria. The branched chain amino acids include leucine, valine and isoleucine.
This disorder is caused by a deficiency of branched chain alpha-keto acid dehydrogenase complex-BCKDC (Annora G. et al). This deficiency leads to accumulation of these three amino acids in blood. Further, an increased level of toxic metabolites of these amino acids is also observed in urine through the sweet odor that they produce as they produce sotolone compound. During birth, infants look normal but with time, they can suffer from severe damages of the brain if medication or treatment is not sought earlier enough. This condition shows dehydration, seizures, ketoacidosis, coma, lethargy and hypoglycemia. Maple syrup urine is genetically classified or it can be through the sequence of its signs and symptoms. It could be divided into intermediate MSUD, Classic severe MSUD, Thiamine responsive MSUD, Intermittent MSUD and E3-Deficient MSUD. Classic severe is the most wide spread and severe form known so far that manifests itself in a newborn at birth. Since this disease is caused by amino acids, to avoid it, people are advised to eat a diet that is less concentrated in proteins.
Argininosuccinic aciduria is also inherited condition that leads to production of high amounts of argininosuccinic acid in urine and blood. It develops from the citric acid cycle where Argininosuccinate lyase (ASL)enzyme is used to breakdown argininosuccinate to arginine, fumarate and dicarboxylic acid.Any mutation of this enzyme results into a reduced enzyme activity.
Argininosuccinate lyase is made of four monomers which are identical. For its catalysis, it has four active sites that help in the catalysis of argininosuccinate catabolism. Fumarate and arginine are formed from the breakdown of argininosuccinate using elimination method. This reaction step is the fourth one in a series of reaction in the urea cycle. At this stage fumarate enters the tricarboxylic acid cycle ( TCA) while arginine yields urea and orthinine. When this enzyme is mutated, it causes argininosuccinic aciduria which is also an autosomal recessive disorder which is not common.This leads to accumulation of compounds in the body which leads to hypothermia, vomiting, lethargy, muscle weakness, liver enlargement hyperventilation, mental retardation and hepatic fibrosis. The condition is managed through reduction of intake food with high protein contents.
Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency is an inherited autosomal disease which is as a result of amino acid catabolism. It actually prevents some fats from being converted to energy. This is mostly experienced during starvation and fasting. It is characterized by hypoglycemia or low blood sugar, lethargy or lack of energy and vomiting.
by bridgettpayseur at 04-27-2013, 08:13 AM
1 comments
Many neurological diseases are caused by misfolded proteins in the brain and along the nervous system. These include Charcot-Marie-Tooth disease, Parkinson’s disease, multiple sclerosis, and Alzheimer’s disease. The misfolfded proteins seem to cause damage to the nervous system and prevent proper functioning of the brain and other parts of the nervous system. These diseases are not reversible, and there are no available treatments to help properly fold the proteins after disease has begun.
Charcot-Marie-Tooth (CMT) is a group of diseases that affect the peripheral nervous system in which the myelin sheaths surrounding the nerve cells are not properly formed. Myelin is a fatty material that is produced by a type of cell called Schwann cells. The myelin acts to insulate the nerve cells, allowing electrical impulses to quickly and easily be conducted throughout the body. Breakdown of the myelin sheath due to CMT results in a decreased ability of the nerve to transmit signals, and leads to degeneration of the nerve. The muscle cell activated by the nerve becomes weakened and atrophied due to lack of use, and this is a hallmark of CMT. CMT is one of the most common inherited neurological disorders, affecting about one in every twenty five hundred people in the United States. CMT is a progressive neuropathy, which means that the condition continues to worsen over time. Although CMT is not fatal in and of itself, it can cause severe effects, limiting mobility and decreasing quality of life.
A common mutation associated with certain types ofCMT is in a protein called P0. P0 seems to act as a molecular glue that holds the myelin around the neuron. If the protein is mutated, then the myelin is not properly attached to myelin. Mutations in P0 have been linked to neuron degeneration and muscle wasting in mice.
Researchers at the University of Buffalo found that when the cell detects defective or misfolded P0 being produced, production of protein is shut off. After the cell has corrected the problem, a protein called Gadd34 causes the cell to resume production of P0. Unfortunately, the Gadd34 causes very high levels of P0 and myelin to be produced, which actually causes more dysfunction in the cell. The excess production of P0 prevents adequate attachment of myelin to the outside of the nerve cell. The researchers found that simply reducing the level of Gadd34 present in the Schwann cells results in increased myelination of the nerve cell. The researchers were able to improve myelin production for nerve cells in both tissue culture and transgenic mouse models.
It will take time and research before this information can be translated into human studies. Researchers will first need to determine if Gadd34 has the same effect on myelin production in human Schwann cells as it does in mouse cells. Because the drug used in tissue culture and in the mouse trials is not approved for human use, the researchers will then need to find or develop a safe molecule that can be given to human volunteers to reduce the level of Gadd34 in the Schwann cells. They will also need to determine the best route of introduction of this molecule. Then, the researchers will need to determine how effectively the rebuilding of the myelin sheath around nerve cells can help restore nerve function. After nerve function is restored, patients would still require physical therapy to help restore muscle function. All of this will take many years to complete, but the prospect of any potential therapy against CMT or other similar neurological diseases is encouraging.
Neuropathies such as CMT are difficult to study, because they may be due to mutations in dozens of genes. The different variations of CMT are also most likely caused by mutations in different genes, and are inherited in different patterns. CMT can be inherited as either a sex-linked disorder or as an autosomal disorder, and can be controlled by either recessive or dominant genes. This diversity of different genetic causes of CMT also means that any potential treatments would need to address all of the potential mutations. One reason Gadd34 is so exciting to researchers is that it could potentially address multiple proteins that are being made incorrectly. Inhibition of Gadd34 could therefore be used to treat multiple types of CMT, as well as other neurothapies.
References:
http://www.sciencedaily.com/releases/201...135037.htm
http://www.ninds.nih.gov/disorders/charc..._tooth.htm
Charcot-Marie-Tooth (CMT) is a group of diseases that affect the peripheral nervous system in which the myelin sheaths surrounding the nerve cells are not properly formed. Myelin is a fatty material that is produced by a type of cell called Schwann cells. The myelin acts to insulate the nerve cells, allowing electrical impulses to quickly and easily be conducted throughout the body. Breakdown of the myelin sheath due to CMT results in a decreased ability of the nerve to transmit signals, and leads to degeneration of the nerve. The muscle cell activated by the nerve becomes weakened and atrophied due to lack of use, and this is a hallmark of CMT. CMT is one of the most common inherited neurological disorders, affecting about one in every twenty five hundred people in the United States. CMT is a progressive neuropathy, which means that the condition continues to worsen over time. Although CMT is not fatal in and of itself, it can cause severe effects, limiting mobility and decreasing quality of life.
A common mutation associated with certain types ofCMT is in a protein called P0. P0 seems to act as a molecular glue that holds the myelin around the neuron. If the protein is mutated, then the myelin is not properly attached to myelin. Mutations in P0 have been linked to neuron degeneration and muscle wasting in mice.
Researchers at the University of Buffalo found that when the cell detects defective or misfolded P0 being produced, production of protein is shut off. After the cell has corrected the problem, a protein called Gadd34 causes the cell to resume production of P0. Unfortunately, the Gadd34 causes very high levels of P0 and myelin to be produced, which actually causes more dysfunction in the cell. The excess production of P0 prevents adequate attachment of myelin to the outside of the nerve cell. The researchers found that simply reducing the level of Gadd34 present in the Schwann cells results in increased myelination of the nerve cell. The researchers were able to improve myelin production for nerve cells in both tissue culture and transgenic mouse models.
It will take time and research before this information can be translated into human studies. Researchers will first need to determine if Gadd34 has the same effect on myelin production in human Schwann cells as it does in mouse cells. Because the drug used in tissue culture and in the mouse trials is not approved for human use, the researchers will then need to find or develop a safe molecule that can be given to human volunteers to reduce the level of Gadd34 in the Schwann cells. They will also need to determine the best route of introduction of this molecule. Then, the researchers will need to determine how effectively the rebuilding of the myelin sheath around nerve cells can help restore nerve function. After nerve function is restored, patients would still require physical therapy to help restore muscle function. All of this will take many years to complete, but the prospect of any potential therapy against CMT or other similar neurological diseases is encouraging.
Neuropathies such as CMT are difficult to study, because they may be due to mutations in dozens of genes. The different variations of CMT are also most likely caused by mutations in different genes, and are inherited in different patterns. CMT can be inherited as either a sex-linked disorder or as an autosomal disorder, and can be controlled by either recessive or dominant genes. This diversity of different genetic causes of CMT also means that any potential treatments would need to address all of the potential mutations. One reason Gadd34 is so exciting to researchers is that it could potentially address multiple proteins that are being made incorrectly. Inhibition of Gadd34 could therefore be used to treat multiple types of CMT, as well as other neurothapies.
References:
http://www.sciencedaily.com/releases/201...135037.htm
http://www.ninds.nih.gov/disorders/charc..._tooth.htm
by bridgettpayseur at 04-27-2013, 01:34 AM
0 comments
Diabetes mellitus is a condition in which the body is not able to properly utilize sugar. There are two types of diabetes mellitus, Type I and Type II. Type I diabetes mellitus is an autoimmune disorder, in which the body’s immune system attacks and destroys insulin producing β cells in the pancreas. Patients with Type I diabetes mellitus require daily insulin injections in order to help their cells take in glucose, and are normally diagnosed with the disease as juveniles. Type II diabetes mellitus is an inability of the body to either make sufficient insulin, or to use it properly. Type II diabetes mellitus usually develops later in life, and may or may not require daily insulin injections to control blood sugar levels.
Long term treatments for diabetes mellitus involve proper nutrition, exercise, daily insulin injections, and medications. In addition, daily monitoring of blood glucose levels is required. This can be necessary many times a day, depending on the severity of disease and how well the patient is controlling his or her blood sugar. While these methods can help a patient control blood glucose levels and stay healthy, there is no treatment available that can replace the defective pancreatic β cells or improve insulin production and usage by the patient. Long term effects of uncontrolled blood glucose can result in heart disease, kidney disease, neuropathy, and circulation problems, and can be life threatening.
Recently, however, a research team at the Harvard Stem Cell Institute, discovered a hormone being produced in the liver that they named betatrophin. The team induced insulin resistance in mice, which caused increased replication of β cells in the pancreas. They searched for upregulated genes in these mice, and found the gene for betatrophin. The betatrophin hormone appeared to help increase the proliferation of β cells to make up for the insulin resistance in the mice. The researchers are particularly excited because the hormone is very specific for β cells, and is very efficient at inducing replication of these cells.
The research team hopes that by injecting volunteers with human betatrophin, they can induce similar replication of β cells in patients with Type II diabetes mellitus. This would increase the amount of insulin being produced, which could help ease some of the symptoms of the disease, and make glucose uptake by other cells more efficient. The betatrophin injections would be required less frequently than insulin injections, possibly as infrequently as once per year. This would be a simple way to improve patient compliance, and decrease the amount of medication needed. The researchers want to begin clinical trials once they have been able to produce sufficient quantities of human betatrophin. Ideally, patients with Type II diabetes mellitus would still continue with a proper diet and exercise regimen, in addition to any treatments, including betatrophin. Combining multiple methods of controlling blood glucose levels would almost certainly be more effective than any one method alone.
The research team also suggested betatrophin as a possible treatment for Type I diabetes mellitus. However, this seems like it might not be as effective as a treatment for Type II diabetes mellitus. Since Type I diabetes mellitus is an autoimmune disorder, the β cells in the pancreas are attacked and destroyed. If there are any β cells present to reproduce after betatrophin treatment, they would all most likely be targeted again by the immune system. The most plausible long term treatment for Type I diabetes mellitus would require the generation of β cells that are not recognized by the host’s immune system. They would have to be free of whatever markers the host immune system is recognizing. In order for this strategy to work, researchers would need to determine how the immune system is recognizing β cells and find a way to engineer cells that are not detected by the immune system. Another option would be therapy to decrease the immune response against β cells, but this could leave the patient susceptible to infection.
While there is still much work that needs to be done to find effective, long term treatments for both Type I and Type II diabetes mellitus, the discovery of betatrophin is very exciting. By teaching the body how to replace β cells in the pancreas, many patients with diabetes and pre-diabetes could be able to better control their blood sugar levels and prevent the harmful long term effects of diabetes.
References:
http://www.nature.com/news/liver-hormone...nt-1.12878
Long term treatments for diabetes mellitus involve proper nutrition, exercise, daily insulin injections, and medications. In addition, daily monitoring of blood glucose levels is required. This can be necessary many times a day, depending on the severity of disease and how well the patient is controlling his or her blood sugar. While these methods can help a patient control blood glucose levels and stay healthy, there is no treatment available that can replace the defective pancreatic β cells or improve insulin production and usage by the patient. Long term effects of uncontrolled blood glucose can result in heart disease, kidney disease, neuropathy, and circulation problems, and can be life threatening.
Recently, however, a research team at the Harvard Stem Cell Institute, discovered a hormone being produced in the liver that they named betatrophin. The team induced insulin resistance in mice, which caused increased replication of β cells in the pancreas. They searched for upregulated genes in these mice, and found the gene for betatrophin. The betatrophin hormone appeared to help increase the proliferation of β cells to make up for the insulin resistance in the mice. The researchers are particularly excited because the hormone is very specific for β cells, and is very efficient at inducing replication of these cells.
The research team hopes that by injecting volunteers with human betatrophin, they can induce similar replication of β cells in patients with Type II diabetes mellitus. This would increase the amount of insulin being produced, which could help ease some of the symptoms of the disease, and make glucose uptake by other cells more efficient. The betatrophin injections would be required less frequently than insulin injections, possibly as infrequently as once per year. This would be a simple way to improve patient compliance, and decrease the amount of medication needed. The researchers want to begin clinical trials once they have been able to produce sufficient quantities of human betatrophin. Ideally, patients with Type II diabetes mellitus would still continue with a proper diet and exercise regimen, in addition to any treatments, including betatrophin. Combining multiple methods of controlling blood glucose levels would almost certainly be more effective than any one method alone.
The research team also suggested betatrophin as a possible treatment for Type I diabetes mellitus. However, this seems like it might not be as effective as a treatment for Type II diabetes mellitus. Since Type I diabetes mellitus is an autoimmune disorder, the β cells in the pancreas are attacked and destroyed. If there are any β cells present to reproduce after betatrophin treatment, they would all most likely be targeted again by the immune system. The most plausible long term treatment for Type I diabetes mellitus would require the generation of β cells that are not recognized by the host’s immune system. They would have to be free of whatever markers the host immune system is recognizing. In order for this strategy to work, researchers would need to determine how the immune system is recognizing β cells and find a way to engineer cells that are not detected by the immune system. Another option would be therapy to decrease the immune response against β cells, but this could leave the patient susceptible to infection.
While there is still much work that needs to be done to find effective, long term treatments for both Type I and Type II diabetes mellitus, the discovery of betatrophin is very exciting. By teaching the body how to replace β cells in the pancreas, many patients with diabetes and pre-diabetes could be able to better control their blood sugar levels and prevent the harmful long term effects of diabetes.
References:
http://www.nature.com/news/liver-hormone...nt-1.12878
by SunilNagpal at 04-27-2013, 01:08 AM
0 comments
The idea of Biopolymers as an alternative to the burgeoning load of Petro-Plastics/Petro-Polymers is not an alien concept for either the scientific community or the layman’s world. It’s well understood that Biopolymers/Bioplastics can debottleneck the scope of utility of plastics and their effect on environment. But, despite this realization and despite the commencement of initiatives in this regard since as early as 1925, not much has been achieved or commercialized till date. This article focuses on highlighting the trends in scientific research and industrial adaptability of Bioplastics, the evolution of concept of Biopolymers in last 8 decades and current status of research on Bioplastics.
Nature offers plethora of Biopolymers, not all of them can be used to replace a polymer as light and as sturdy as Polythene/Polystyrene. Starch, one of the most abundant Biopolymers, was first considered a probable solution to making a biodegradable plastic, but it’s structure and chemical nature is so deviant from the thermoplastics that neither the form nor the properties of thermoplastics could be replicated in starch based plastic. Efforts to create a blend of starch and polythene were also made but only to reach at a disappointing conclusion that though starch component tends to degrade and makes the plastic porous, the residual material is rendered non-degradable and unaltered. Evidently, need of the hour was to find a radically new polymer, whose characteristics could be a replica of the over-adapted thermoplastics, except for their degradability. Readers might be intrigued to imagine the world before crude oil based thermoplastics came into being, and to satiate your curiosity it was none other than Bioploymers only that were abundantly used in day to day applications. Plant resins like Amber and Shellac were materials of choice for making seals, crafts, woodwork finishes etc. Later, John Wesley Hyatt(1869) came up with a bio-plastic to give shiny finishing to billiard balls, which with further research became so popular in the market that from Packaging to Photographic films, everything was based on the polymer discovered by Hyatt. Today we know that polymer as Cellulose (the most abundant Biopolymer on Earth). Infact, cellulose remains one of those ancient Biopolymers, that has retained its industrial usability despite the overhaul of petroleum based polymers. The retention of cellulose can be attributed to its biocompatibility, ability to cast into films and self-sealing nature which comes handy in food packaging and medical devices industry. But, when compared to thermoplastics like Polythene, cellulose still falls short in density range, chemical resistance, molecular weight, ease of production and most importantly cost of production, telling us why the world is obsessed with Polythene/Polyurethane/Polystyrene!
A revolutionary discovery was finally made in 1925 by Lemoigne of Pasteur Institute, France, who observed granular storage materials inside Bacillus megaterium, chemically recognized as a polymer of 3-hydroxybutyrate and popularized as PHB (Poly-(3-hyrdroxybutyrate)). The granules exhibited extra-ordinary properties of water insolubility, resistance to hydrolytic degradation, high tensile strength, high melting point (around 180 degree Celsius), non-toxic nature and most importantly biodegradability. The production of these granules was induced in the bacteria only under stressed environments like nutrient limitation or oxygen stress to act as an energy reserve for prospective starving conditions. But by the same time, petroleum based thermoplastics became popularized owing to their ease of production and abundant availability. It was in the latter half of the 20th century that with the rising concerns about the accumulating plastics and depleting oil reserves, focus shifted towards finding alternatives to the polluting, non-biodegrading and non-renewable resources. By 1974, discovery of monomers other than 3-HB in the granules of Bacillus megaterium growing on different substrates was established. PHB became a member of big family of Polymers termed as PHAs (polyhydroxyalkanoates) thereby making PHAs the most potent candidates for replacing the wide variety of Polythenes (LDPE, MDPE, HDPE, ULDPE etc). Discovery of PHAs in many other bacterial species like Cupriavidus spp., Psedomonas spp. further triggered the research in this area. Such was the surge in the research that Imperial Chemical Industries (UK) came out with the first commercial PHB based bioplastic under the trade name of BIOPOL(TM) in 1980. BIOPOL was produced through a Fed Batch culture of Cupriavidus necator and was a copolymer of 3-hydroxybutyrate and 3-hydroxyvalerate. But the production of BIOPOL is very limited and application till date is confined to surgical sutures, surgical meshes and medical implantable devices. The anarchy of Polybags as we all know, still prevails.
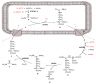
The biggest reason behind the limited acceptability of PHA based bioplastics despite their striking similarities to petroleum based thermoplastics is their cost of production. And this in turn is caused by the chain of steps involved in producing and recovering PHAs. A basic flow diagram of steps involved in producing PHAs is depicted below:
This multitude of steps for obtaining PHA makes the entire process unavoidably expensive, unless the production levels at the upstream (Fermentation) level are extraordinarily high. Infact, that’s what the focus of current research has been improvement of upstream stages of PHA production. Genetic modification of strains, different design of reactors, different substrates and different operating conditions have been tried by various groups of scientists across the globe to meet the demand for a successful commercial set-up. Recently (March 2013), US National Science Foundation announced funding for Biopolymers and Biocomposites Centre at Iowa State University to catalyze the research in this area. Similarly, Department of Biochemical Engineering, Indian Institute of Technology, Delhi received a generous grant of INR 42,000,000 to develop a Centre for Excellence in Biopolymers’ Research from Govt. of India. The research is on the go in different parts of the world at an equally active rate, and the time should not be long when we may dump the plastic bags and bottles in the same bin as our kitchen waste.
Nature offers plethora of Biopolymers, not all of them can be used to replace a polymer as light and as sturdy as Polythene/Polystyrene. Starch, one of the most abundant Biopolymers, was first considered a probable solution to making a biodegradable plastic, but it’s structure and chemical nature is so deviant from the thermoplastics that neither the form nor the properties of thermoplastics could be replicated in starch based plastic. Efforts to create a blend of starch and polythene were also made but only to reach at a disappointing conclusion that though starch component tends to degrade and makes the plastic porous, the residual material is rendered non-degradable and unaltered. Evidently, need of the hour was to find a radically new polymer, whose characteristics could be a replica of the over-adapted thermoplastics, except for their degradability. Readers might be intrigued to imagine the world before crude oil based thermoplastics came into being, and to satiate your curiosity it was none other than Bioploymers only that were abundantly used in day to day applications. Plant resins like Amber and Shellac were materials of choice for making seals, crafts, woodwork finishes etc. Later, John Wesley Hyatt(1869) came up with a bio-plastic to give shiny finishing to billiard balls, which with further research became so popular in the market that from Packaging to Photographic films, everything was based on the polymer discovered by Hyatt. Today we know that polymer as Cellulose (the most abundant Biopolymer on Earth). Infact, cellulose remains one of those ancient Biopolymers, that has retained its industrial usability despite the overhaul of petroleum based polymers. The retention of cellulose can be attributed to its biocompatibility, ability to cast into films and self-sealing nature which comes handy in food packaging and medical devices industry. But, when compared to thermoplastics like Polythene, cellulose still falls short in density range, chemical resistance, molecular weight, ease of production and most importantly cost of production, telling us why the world is obsessed with Polythene/Polyurethane/Polystyrene!
A revolutionary discovery was finally made in 1925 by Lemoigne of Pasteur Institute, France, who observed granular storage materials inside Bacillus megaterium, chemically recognized as a polymer of 3-hydroxybutyrate and popularized as PHB (Poly-(3-hyrdroxybutyrate)). The granules exhibited extra-ordinary properties of water insolubility, resistance to hydrolytic degradation, high tensile strength, high melting point (around 180 degree Celsius), non-toxic nature and most importantly biodegradability. The production of these granules was induced in the bacteria only under stressed environments like nutrient limitation or oxygen stress to act as an energy reserve for prospective starving conditions. But by the same time, petroleum based thermoplastics became popularized owing to their ease of production and abundant availability. It was in the latter half of the 20th century that with the rising concerns about the accumulating plastics and depleting oil reserves, focus shifted towards finding alternatives to the polluting, non-biodegrading and non-renewable resources. By 1974, discovery of monomers other than 3-HB in the granules of Bacillus megaterium growing on different substrates was established. PHB became a member of big family of Polymers termed as PHAs (polyhydroxyalkanoates) thereby making PHAs the most potent candidates for replacing the wide variety of Polythenes (LDPE, MDPE, HDPE, ULDPE etc). Discovery of PHAs in many other bacterial species like Cupriavidus spp., Psedomonas spp. further triggered the research in this area. Such was the surge in the research that Imperial Chemical Industries (UK) came out with the first commercial PHB based bioplastic under the trade name of BIOPOL(TM) in 1980. BIOPOL was produced through a Fed Batch culture of Cupriavidus necator and was a copolymer of 3-hydroxybutyrate and 3-hydroxyvalerate. But the production of BIOPOL is very limited and application till date is confined to surgical sutures, surgical meshes and medical implantable devices. The anarchy of Polybags as we all know, still prevails.
The biggest reason behind the limited acceptability of PHA based bioplastics despite their striking similarities to petroleum based thermoplastics is their cost of production. And this in turn is caused by the chain of steps involved in producing and recovering PHAs. A basic flow diagram of steps involved in producing PHAs is depicted below:
This multitude of steps for obtaining PHA makes the entire process unavoidably expensive, unless the production levels at the upstream (Fermentation) level are extraordinarily high. Infact, that’s what the focus of current research has been improvement of upstream stages of PHA production. Genetic modification of strains, different design of reactors, different substrates and different operating conditions have been tried by various groups of scientists across the globe to meet the demand for a successful commercial set-up. Recently (March 2013), US National Science Foundation announced funding for Biopolymers and Biocomposites Centre at Iowa State University to catalyze the research in this area. Similarly, Department of Biochemical Engineering, Indian Institute of Technology, Delhi received a generous grant of INR 42,000,000 to develop a Centre for Excellence in Biopolymers’ Research from Govt. of India. The research is on the go in different parts of the world at an equally active rate, and the time should not be long when we may dump the plastic bags and bottles in the same bin as our kitchen waste.
by bridgettpayseur at 04-26-2013, 11:53 PM
1 comments
For more than twenty five years, researchers have been trying to find a way to stop the spread of the human immunodeficiency virus (HIV), the causative agent of acquired immunodeficiency syndrome (AIDS). The virus has proven difficult to stop for many reasons. HIV is a retrovirus. This means it contains a genome composed of RNA, which is then reverse transcribed into DNA once the virus has entered the host cell. Once the DNA has been made, it is integrated into the host cell’s genome. This means that whenever the host cell replicates, the viral genome replicates as well. Once the viral DNA has been inserted into the genome, the host cell’s transcription and translation machinery will produce viral proteins, reproducing the virus. The newly formed viral particles will then leave the host cell, without killing it, to infect new cells.
A clinical trial of an HIV vaccine which began in 2009 was recently stopped. Twenty five hundred participants in the trial received either a placebo injection, or an experimental HIV vaccine. A safety review recently showed that slightly more patients who received the vaccine were infected with HIV than patients who received the placebo. In addition, patients who received the vaccine did not have lower viral loads compared to patients who received the placebo.
The vaccine being studied was based on an adenoviral vector. Adenovirus is a cause of the common cold, and has been studied in bioengineering as a vector for vaccination and gene therapy treatments. The adenovirus was engineered to produce HIV proteins, and then train CD8+ T cells to recognize virus infected cells and destroy them. The study used a prime-boost strategy, in which volunteers were given two doses of the vaccine to prime the immune system, and then produce a stronger, secondary immune response. While the failure of the study is disappointing, it is not entirely unexpected. Over the past twenty five years, there have been HIV vaccine trials. A recent study in Thailand showed some promise, but the study needs to be expanded on to find a more effective regimen. Other HIV vaccine trials have not been as successful. Indeed, many previous trials have been halted due to poor performance of the vaccine.
There are many reasons why it has been so difficult for scientists to produce a functional HIV vaccine. A strong CD4+ T cell response early during HIV infection has been shown to enhance the pathogenicity of the virus, resulting in increased viral load and more severe disease. CD8+ T cells are a popular target of vaccine design, as they are able to directly recognize and kill virus infected cells. However, because HIV is a retrovirus, it is highly susceptible to mutations. These mutations occur from high error rate of the reverse transcriptase protein. The mutations may alter the viral proteins enough that they are not recognized by the CD8+ T cells, and can escape the immune response. In fact, the mutation rate of HIV is so high, that many patients are infected with many different subspecies of virus. In addition, there are many different strains and subtypes of HIV, and a vaccine specific to one strain may not elicit an immune response against other strains. Lastly, because many HIV vaccines currently in development utilize live vectors, such as a virus or bacteria, it is possible that a patient might already have encountered that vector, and have an immune response against it. If this happens, the patient’s immune system would destroy the vector before it has a chance to induce an effective anti-HIV immune response.
Currently, research indicates that the production of broadly neutralizing antibodies may be the best route to take when exploring potential HIV vaccines. Broadly neutralizing antibodies have been found in some long term non-progressors, which are patients who have been infected with the virus but have been able to maintain low to undetectable viral loads without antiretroviral therapy. The broadly neutralizing antibodies recognize portions of protein that are highly conserved throughout many HIV strains. Because these proteins are important for viral binding and entry into the host cell, they do not mutate at a high rate, either. Scientists are studying ways to induce production of these broadly neutralizing antibodies in hopes of providing protection against a large range of HIV strains and subtypes. In the end, a combination of broadly neutralizing antibodies, along with strong CD8+ T cell responses, will probably be the most effective method for preventing, or at least controlling, HIV infection with a vaccine.
References:
http://www.cbsnews.com/8301-204_162-5758...nfections/
http://www.dailymail.co.uk/health/articl...paign=1490
A clinical trial of an HIV vaccine which began in 2009 was recently stopped. Twenty five hundred participants in the trial received either a placebo injection, or an experimental HIV vaccine. A safety review recently showed that slightly more patients who received the vaccine were infected with HIV than patients who received the placebo. In addition, patients who received the vaccine did not have lower viral loads compared to patients who received the placebo.
The vaccine being studied was based on an adenoviral vector. Adenovirus is a cause of the common cold, and has been studied in bioengineering as a vector for vaccination and gene therapy treatments. The adenovirus was engineered to produce HIV proteins, and then train CD8+ T cells to recognize virus infected cells and destroy them. The study used a prime-boost strategy, in which volunteers were given two doses of the vaccine to prime the immune system, and then produce a stronger, secondary immune response. While the failure of the study is disappointing, it is not entirely unexpected. Over the past twenty five years, there have been HIV vaccine trials. A recent study in Thailand showed some promise, but the study needs to be expanded on to find a more effective regimen. Other HIV vaccine trials have not been as successful. Indeed, many previous trials have been halted due to poor performance of the vaccine.
There are many reasons why it has been so difficult for scientists to produce a functional HIV vaccine. A strong CD4+ T cell response early during HIV infection has been shown to enhance the pathogenicity of the virus, resulting in increased viral load and more severe disease. CD8+ T cells are a popular target of vaccine design, as they are able to directly recognize and kill virus infected cells. However, because HIV is a retrovirus, it is highly susceptible to mutations. These mutations occur from high error rate of the reverse transcriptase protein. The mutations may alter the viral proteins enough that they are not recognized by the CD8+ T cells, and can escape the immune response. In fact, the mutation rate of HIV is so high, that many patients are infected with many different subspecies of virus. In addition, there are many different strains and subtypes of HIV, and a vaccine specific to one strain may not elicit an immune response against other strains. Lastly, because many HIV vaccines currently in development utilize live vectors, such as a virus or bacteria, it is possible that a patient might already have encountered that vector, and have an immune response against it. If this happens, the patient’s immune system would destroy the vector before it has a chance to induce an effective anti-HIV immune response.
Currently, research indicates that the production of broadly neutralizing antibodies may be the best route to take when exploring potential HIV vaccines. Broadly neutralizing antibodies have been found in some long term non-progressors, which are patients who have been infected with the virus but have been able to maintain low to undetectable viral loads without antiretroviral therapy. The broadly neutralizing antibodies recognize portions of protein that are highly conserved throughout many HIV strains. Because these proteins are important for viral binding and entry into the host cell, they do not mutate at a high rate, either. Scientists are studying ways to induce production of these broadly neutralizing antibodies in hopes of providing protection against a large range of HIV strains and subtypes. In the end, a combination of broadly neutralizing antibodies, along with strong CD8+ T cell responses, will probably be the most effective method for preventing, or at least controlling, HIV infection with a vaccine.
References:
http://www.cbsnews.com/8301-204_162-5758...nfections/
http://www.dailymail.co.uk/health/articl...paign=1490
by arman at 04-26-2013, 07:57 PM
0 comments
Huy guys!
My name is Arman. Its my first time to post on this forum,just want to make some friends here.if its not allowed to post on this board,please delete this thread.Nice to see you guys here. Guys about 2 years ago My friend suffered from a big problem. He had plantar wart (verruca) surgery to remove a plantar wart from the ball of her foot. He did know about the risk of scarring before the procedure but he had enough of the wart being in my foot for 2/3 years. Now, 2 months later, the ball of his foot is virtually painless to walk on, but when he apply a lot of pressure to the ball of his foot, or when he press it hard against the floor or the wall, he feel some slight pain. The surgery site itself looks good, But friend's I've read that it takes up to a year for a scar to fully develop, so does that mean the pain will stop after that time.......?
My name is Arman. Its my first time to post on this forum,just want to make some friends here.if its not allowed to post on this board,please delete this thread.Nice to see you guys here. Guys about 2 years ago My friend suffered from a big problem. He had plantar wart (verruca) surgery to remove a plantar wart from the ball of her foot. He did know about the risk of scarring before the procedure but he had enough of the wart being in my foot for 2/3 years. Now, 2 months later, the ball of his foot is virtually painless to walk on, but when he apply a lot of pressure to the ball of his foot, or when he press it hard against the floor or the wall, he feel some slight pain. The surgery site itself looks good, But friend's I've read that it takes up to a year for a scar to fully develop, so does that mean the pain will stop after that time.......?
by bridgettpayseur at 04-26-2013, 12:32 PM
0 comments
Ribonucleic acid (RNA) is generally known for its role in the production of protein. Messenger RNA (mRNA) acts as a middle-man between DNA and the protein building ribosomal RNA and transfer RNA molecules. mRNA is transcribed from the directions provided by DNA. It then moves from the nucleus of the cell to the ribosomes, which are located in the cytoplasm, to be used as a template for protein translation. This process of going from DNA to RNA to protein is termed the central dogma of molecular biology, and describes some of the most important processes common to all living organisms.
However, some short stretches of single stranded RNA, termed micro RNA (miRNA) are also involved in silencing gene expression. miRNA functions by finding long single strands of RNA in the cytoplasm. When the miRNA is able to complimentary base pair with a molecule of single stranded RNA, it prevents the gene from being expressed. miRNA mediated gene silencing can occur by either targeting the RNA for degradation, or by preventing translational machinery from accessing the RNA and making protein. This is a defense mechanism in the cell that helps protect against viral infections.
Gene silencing using miRNAs is a popular area of study, with high hopes for treating diseases and as a possible gene therapy tool. In many diseases, the expression of a particular gene may either be increased or decreased. Using gene silencing could provide a method to control the expression of these vital genes, thereby limiting symptoms and disease severity. In addition to potential therapeutic use, gene silencing is often used as a tool in scientific research, to determine cellular responses in the absence of a particular gene. miRNA are transferred into cells to help determine the function of a specific gene. Scrambled miRNA is an easily available negative control that allows the researchers to determine if the transfer protocol has affected the cells in any way. Gene silencing is an efficient, easily controlled method to inhibit gene expression in both primary cells as well as immortalized cell lines.
It is not know how precisely gene silencing works in the cell. It is suspected that binding of miRNA to single stranded RNA may simply block the ribosome, the organelle that builds proteins, from binding to the messenger RNA, thus blocking translation. However, targeted degredation of RNA is less well understood. Researchers do not yet understand how the miRNA brings machinery to the RNA to break it down. It is known that certain molecules seem to help cut up the single stranded RNA, which prevents translation of the gene.
While the general mechanism for gene silencing is understood, the location in the cell and specific details of gene silencing by miRNA has only been recently examined. Researchers at the University of California at Riverside have recently identified the membrane of the endoplasmic reticulum (ER) as the location of gene silencing. The ER is a network of membranes within the cell that is used for transport and production of organic molecules such as proteins and lipids. The researchers determined that miRNA mediated gene silencing requires a membrane protein called AMP-1, which is found in both the cell membrane as well as the membrane of the ER in both plant and animal cells. This was a surprising location for gene silencing to occur. The researchers hypothesize that most gene silencing occurs in the rough ER, which contains protein building ribosomes. Indeed, many secretory proteins are built in the rough ER so they can be packaged in transport vesicles and sent to their final destination. In addition, the protein AMP-1 is found primarily in the membrane of the rough ER.
The researchers obtained this data from studies conducted using plant cells; however, since AMP-1 is found in both plant and animal cells, they are confident the results will be similar in plant cells. Knowing where to look in the cell for miRNA mediated gene silencing will allow researchers to further dissect the mechanisms of gene silencing. The team plans to determine how the ER is involved in miRNA mediated RNA degredation, as well as determining how miRNA is targeted to the ER. This information will be valuable as more therapies are designed using gene silencing in the treatment of diseases.
References:
http://www.sciencedaily.com/releases/201...132656.htm
However, some short stretches of single stranded RNA, termed micro RNA (miRNA) are also involved in silencing gene expression. miRNA functions by finding long single strands of RNA in the cytoplasm. When the miRNA is able to complimentary base pair with a molecule of single stranded RNA, it prevents the gene from being expressed. miRNA mediated gene silencing can occur by either targeting the RNA for degradation, or by preventing translational machinery from accessing the RNA and making protein. This is a defense mechanism in the cell that helps protect against viral infections.
Gene silencing using miRNAs is a popular area of study, with high hopes for treating diseases and as a possible gene therapy tool. In many diseases, the expression of a particular gene may either be increased or decreased. Using gene silencing could provide a method to control the expression of these vital genes, thereby limiting symptoms and disease severity. In addition to potential therapeutic use, gene silencing is often used as a tool in scientific research, to determine cellular responses in the absence of a particular gene. miRNA are transferred into cells to help determine the function of a specific gene. Scrambled miRNA is an easily available negative control that allows the researchers to determine if the transfer protocol has affected the cells in any way. Gene silencing is an efficient, easily controlled method to inhibit gene expression in both primary cells as well as immortalized cell lines.
It is not know how precisely gene silencing works in the cell. It is suspected that binding of miRNA to single stranded RNA may simply block the ribosome, the organelle that builds proteins, from binding to the messenger RNA, thus blocking translation. However, targeted degredation of RNA is less well understood. Researchers do not yet understand how the miRNA brings machinery to the RNA to break it down. It is known that certain molecules seem to help cut up the single stranded RNA, which prevents translation of the gene.
While the general mechanism for gene silencing is understood, the location in the cell and specific details of gene silencing by miRNA has only been recently examined. Researchers at the University of California at Riverside have recently identified the membrane of the endoplasmic reticulum (ER) as the location of gene silencing. The ER is a network of membranes within the cell that is used for transport and production of organic molecules such as proteins and lipids. The researchers determined that miRNA mediated gene silencing requires a membrane protein called AMP-1, which is found in both the cell membrane as well as the membrane of the ER in both plant and animal cells. This was a surprising location for gene silencing to occur. The researchers hypothesize that most gene silencing occurs in the rough ER, which contains protein building ribosomes. Indeed, many secretory proteins are built in the rough ER so they can be packaged in transport vesicles and sent to their final destination. In addition, the protein AMP-1 is found primarily in the membrane of the rough ER.
The researchers obtained this data from studies conducted using plant cells; however, since AMP-1 is found in both plant and animal cells, they are confident the results will be similar in plant cells. Knowing where to look in the cell for miRNA mediated gene silencing will allow researchers to further dissect the mechanisms of gene silencing. The team plans to determine how the ER is involved in miRNA mediated RNA degredation, as well as determining how miRNA is targeted to the ER. This information will be valuable as more therapies are designed using gene silencing in the treatment of diseases.
References:
http://www.sciencedaily.com/releases/201...132656.htm
by bridgettpayseur at 04-26-2013, 01:50 AM
2 comments
Finding a sustainable energy source for the rapidly growing needs of the population is an important area of research in science. Current consumption of fossil fuels is not sustainable in the long run, and the combustion of these fuels causes the release of carbon dioxide and other greenhouse gases, which can harm the environment. Even providing energy for electric cars requires the burning of fossil fuels, such as coal, in order to produce electricity. Although the actual machine does not produce as much pollution as a normal car, the production of energy to be used for the electric car does result in the production of greenhouse gasses and other pollutants. Clean, alternative energy sources, include wind and solar power, are gaining in popularity, but they have not been scaled up sufficiently to meet the needs of the growing population. While research continues to find cleaner energy sources, a more immediate solution might involve production of biofuels, petroleum products derived from organic sources.
Unfortunately, many of the biofuels currently being produced may cause problems for the environment. The production of these biofuels is not straightforward either, and requires many steps to get a final product that can be used. They require treatment with petroleum products, which means they may not be sustainable in the long run. In addition, growing corn crops to be used for biofuel production is causing increased costs for food, and increasing the land required for farming. The amount of land available to produce sufficient corn for both biofuel production and food is not adequate. Although many have suggested plant sources that grow more quickly that corn, require less space to grow, and will not affect the food supply, these sources have not been utilized extensively for biofuel production. Corn still remains the predominant choice for creating biofuels. Lastly, biofuels may not be structurally similar to conventional petroleum products, which would require upgrades to existing machinery in order for the fuel to be usable. For these reasons, other sources of biofuel are being investigated by energy scientists.
Recently, researchers at the University of Exeter, in collaboration with Shell, have engineered a strain of E. coli bacteria that can produce diesel fuel. This fuel is very similar in composition to traditional diesel fuel, and does not require treatment with petroleum products. Because it is so similar to traditional diesel fuel, systems that use diesel would not need to be upgraded in order to be able to use the fuel. This is a huge benefit over other biofuels, such as those produced from corn ethanol, because they do not need to be mixed with petroleum. The diesel biofuel produced by the E. coli could simply replace the conventional diesel fuel currently in use.
E. coli and other bacteria can transform organic molecules such as sugar into lipids which are inserted into the cell membrane. Lipids and diesel fuel have very similar structures. They are long chains of hydrogen and carbon, termed hydrocarbon molecules. The process of making lipids in E. coli has been modified by the researchers so that instead of producing normal lipids, the bacteria produce diesel fuel. So far, the researchers only have a small number of bacteria producing small quantities of the diesel. However, bacteria are often used in large scale production of many pharmaceuticals. Increasing the number of bacteria producing diesel and the yield would be a similar process. Once the researchers have determined that the diesel fuel being produced is adequate to be used in vehicles and industrial settings, scaling up production should be a straightforward process.
As more people become aware of the damages to the environment traditional energy sources cause, the development of cleaner, more sustainable energy becomes more important. As economies around the world begin to recover from the recessions of a few years ago, citizens will most likely be buying more products and traveling more, which will require more energy. Sustainable energy sources will become more important as demand increases, and supply decreases. In addition, laboratory produced diesel fuel and other clean, sustainable energy sources will enhance economic recovery, as fuel and energy prices will be less dynamic. The field of energy science is growing rapidly, to help keep the energy supply sufficient for human and technological growth.
References:
http://www.sciencedaily.com/releases/201...154911.htm
Unfortunately, many of the biofuels currently being produced may cause problems for the environment. The production of these biofuels is not straightforward either, and requires many steps to get a final product that can be used. They require treatment with petroleum products, which means they may not be sustainable in the long run. In addition, growing corn crops to be used for biofuel production is causing increased costs for food, and increasing the land required for farming. The amount of land available to produce sufficient corn for both biofuel production and food is not adequate. Although many have suggested plant sources that grow more quickly that corn, require less space to grow, and will not affect the food supply, these sources have not been utilized extensively for biofuel production. Corn still remains the predominant choice for creating biofuels. Lastly, biofuels may not be structurally similar to conventional petroleum products, which would require upgrades to existing machinery in order for the fuel to be usable. For these reasons, other sources of biofuel are being investigated by energy scientists.
Recently, researchers at the University of Exeter, in collaboration with Shell, have engineered a strain of E. coli bacteria that can produce diesel fuel. This fuel is very similar in composition to traditional diesel fuel, and does not require treatment with petroleum products. Because it is so similar to traditional diesel fuel, systems that use diesel would not need to be upgraded in order to be able to use the fuel. This is a huge benefit over other biofuels, such as those produced from corn ethanol, because they do not need to be mixed with petroleum. The diesel biofuel produced by the E. coli could simply replace the conventional diesel fuel currently in use.
E. coli and other bacteria can transform organic molecules such as sugar into lipids which are inserted into the cell membrane. Lipids and diesel fuel have very similar structures. They are long chains of hydrogen and carbon, termed hydrocarbon molecules. The process of making lipids in E. coli has been modified by the researchers so that instead of producing normal lipids, the bacteria produce diesel fuel. So far, the researchers only have a small number of bacteria producing small quantities of the diesel. However, bacteria are often used in large scale production of many pharmaceuticals. Increasing the number of bacteria producing diesel and the yield would be a similar process. Once the researchers have determined that the diesel fuel being produced is adequate to be used in vehicles and industrial settings, scaling up production should be a straightforward process.
As more people become aware of the damages to the environment traditional energy sources cause, the development of cleaner, more sustainable energy becomes more important. As economies around the world begin to recover from the recessions of a few years ago, citizens will most likely be buying more products and traveling more, which will require more energy. Sustainable energy sources will become more important as demand increases, and supply decreases. In addition, laboratory produced diesel fuel and other clean, sustainable energy sources will enhance economic recovery, as fuel and energy prices will be less dynamic. The field of energy science is growing rapidly, to help keep the energy supply sufficient for human and technological growth.
References:
http://www.sciencedaily.com/releases/201...154911.htm
by bridgettpayseur at 04-25-2013, 12:40 AM
0 comments
Injuries to the nervous system, from trauma, stroke, or other causes, are nearly impossible to cure with current medical technology. Cells of the nervous system are quiescent, meaning that they do not replicate. If cells are damaged, they cannot be replaced. While therapy can help restore some function for the patient, the injury is considered permanent. One major hope for restoring cells in the nervous system after injury is stem cell therapy. Stem cells that have matured into nerve cells could be used to replace injured nerve cells and repair the injury.
Obtaining stem cells that have the ability to mature into nerve cells, and then producing the right conditions for maturation, are among the challenges scientists and clinicians face in order to begin testing stem cell therapy in patients. Indeed, producing the correct conditions in vitro to help stem cells properly mature into the correct type of cell is a major focus of research. Even when scientists are able to properly mature stem cells into nerve cells, concern arise regarding patient immune responses against donor cells. Using a the patient’s own stem cells to develop new nerve cells would remove the risk of immune rejection from transplantation.
In an accidental discovery, researchers at the Scripps Research Institute found an antibody that could induce bone marrow stem cells to develop into a nerve cell progenitor. The researchers were testing a panel of antibodies to find one capable of increasing growth in the bone marrow stem cells. The surprise discovery was very welcome by the scientists. Normally, antibodies in research are used to locate specific markers on cells, and act as labels. At times, antibody binding to a receptor on the surface of the cell has been known to activate the receptor, and cause changes in the cell. By screening a large library of antibodies against various receptors on the surface of bone marrow stem cells, the researchers unexpectedly found a method for producing nerve cells.
The researchers were screening a panel of antibodies that could recognize a specific growth-factor receptor on bone marrow stem cells. By activating this receptor, scientists can increase production of white blood cells to help mitigate the cytotoxic effects of chemotherapy in cancer patients. When researchers began testing the antibody mentioned above in culture, they noticed that the maturing stem cells became long, thin, and attached to the petri dish. They then tested these maturing cells for markers found on neural cells, and were surprised to learn that they had indeed induced maturation of neural progenitors.
The researchers are not sure why the antibody against the growth factor receptor caused the bone marrow stem cells to mature into neural progenitors. They suggest that the way the antibody binds to the receptor may have an effect on how the cell responds. Drug manufacturers have seen in recent years that how a receptor is bound by a product can have as much of an effect as what receptor is bound, and are beginning research to determine how these minor differences can be utilized and controlled.
While there have been some labs that have had success producing neural progenitor cells from bone marrow derived stem cells, the process is very difficult. First, the bone marrow derived stem cells must be reprogrammed to a more embryonic-like state that is believed to be more amenable to differentiation into various mature cell types. Then, the embryonic-like stem cells must be treated with the proper factors to induce maturation into the desired cell type. The results described above, utilizing antibody, appear to be the first demonstration of bone marrow derived stem cells being directly differentiated into neural progenitor cells. These results could dramatically simplify proposed stem cell therapies. The conventional protocol suggested for stem cell therapy involves removing bone marrow stem cells from a patient, maturing them in vitro into the proper type of cell, and then injecting the mature cells back into the patient. With the new discovery, it might be possible to inject an antibody in a patient. The antibody would help mature some of the bone marrow cells into neural progenitors, which could then migrate to sites of injury and help repair damage. This protocol would be much simpler and less expensive, and possibly be available to a larger number of patients.
References:
http://www.sciencedaily.com/releases/201...154756.htm
Obtaining stem cells that have the ability to mature into nerve cells, and then producing the right conditions for maturation, are among the challenges scientists and clinicians face in order to begin testing stem cell therapy in patients. Indeed, producing the correct conditions in vitro to help stem cells properly mature into the correct type of cell is a major focus of research. Even when scientists are able to properly mature stem cells into nerve cells, concern arise regarding patient immune responses against donor cells. Using a the patient’s own stem cells to develop new nerve cells would remove the risk of immune rejection from transplantation.
In an accidental discovery, researchers at the Scripps Research Institute found an antibody that could induce bone marrow stem cells to develop into a nerve cell progenitor. The researchers were testing a panel of antibodies to find one capable of increasing growth in the bone marrow stem cells. The surprise discovery was very welcome by the scientists. Normally, antibodies in research are used to locate specific markers on cells, and act as labels. At times, antibody binding to a receptor on the surface of the cell has been known to activate the receptor, and cause changes in the cell. By screening a large library of antibodies against various receptors on the surface of bone marrow stem cells, the researchers unexpectedly found a method for producing nerve cells.
The researchers were screening a panel of antibodies that could recognize a specific growth-factor receptor on bone marrow stem cells. By activating this receptor, scientists can increase production of white blood cells to help mitigate the cytotoxic effects of chemotherapy in cancer patients. When researchers began testing the antibody mentioned above in culture, they noticed that the maturing stem cells became long, thin, and attached to the petri dish. They then tested these maturing cells for markers found on neural cells, and were surprised to learn that they had indeed induced maturation of neural progenitors.
The researchers are not sure why the antibody against the growth factor receptor caused the bone marrow stem cells to mature into neural progenitors. They suggest that the way the antibody binds to the receptor may have an effect on how the cell responds. Drug manufacturers have seen in recent years that how a receptor is bound by a product can have as much of an effect as what receptor is bound, and are beginning research to determine how these minor differences can be utilized and controlled.
While there have been some labs that have had success producing neural progenitor cells from bone marrow derived stem cells, the process is very difficult. First, the bone marrow derived stem cells must be reprogrammed to a more embryonic-like state that is believed to be more amenable to differentiation into various mature cell types. Then, the embryonic-like stem cells must be treated with the proper factors to induce maturation into the desired cell type. The results described above, utilizing antibody, appear to be the first demonstration of bone marrow derived stem cells being directly differentiated into neural progenitor cells. These results could dramatically simplify proposed stem cell therapies. The conventional protocol suggested for stem cell therapy involves removing bone marrow stem cells from a patient, maturing them in vitro into the proper type of cell, and then injecting the mature cells back into the patient. With the new discovery, it might be possible to inject an antibody in a patient. The antibody would help mature some of the bone marrow cells into neural progenitors, which could then migrate to sites of injury and help repair damage. This protocol would be much simpler and less expensive, and possibly be available to a larger number of patients.
References:
http://www.sciencedaily.com/releases/201...154756.htm
by bridgettpayseur at 04-24-2013, 11:34 AM
0 comments
Current cancer treatments involve the administration of chemotherapeutic drugs, which act by killing all rapidly proliferating cells. This process, however, results in the killing of many healthy cells in the body, including blood cells, skin cells, and others. This results in very unpleasant side effects, including gastrointestinal problems, decreased ability to fight infections, hair loss, nausea, and skin sensitivities. The treatment options for cancer have not improved over the past several years, so more research into less toxic treatments is desperately needed. In addition to severe side effects, chemotherapy is not always effective at killing all of the cancer cells, in part because it cannot effectively penetrate solid tumors. In addition, many cancer cells have developed mutations that provide protection from such drugs. Cancer cells can increase or decrease the expression of certain receptors on the surface of the cell so that they appear invisible to both drugs and the immune system.
Using attenuated viruses to enhance the effects of chemotherapy drugs has been studied in the past. The virus helps the chemotherapy work more effectively against the cancer cell by weakening the cell so it is more easily killed by the treatment. Adenovirus, which is a causative agent of the common cold, has been studied in many forms for treating cancer. Some genetically engineered strains of adenovirus have been used to infect cancer cells, thereby making them more susceptible to the chemotherapeutics. In addition, adenovirus has been used as a vector for gene therapy, delivering genes to cancer cells that can help remove the cancer from the patient.
Cancer cells tend have deficiencies in the innate immune response that helps protect them from viral infection. This makes cancer cells more susceptible to viral infection than normal, noncancerous cells. Knowing this, researchers wanted to determine if a fast acting virus could selectively infect and kill cancer cells, without harming normal cells. The researcher team first infected melanoma cancer cells in vitro with several strains of a virus called Vesicular Stomatits Virus (VSV). The melanoma cells were efficiently infected and killed by most strains of the virus studied, some of which were able to kill nearly 100% of the melanoma cells. In contrast, healthy melanocytes were not infected as efficiently by VSV. The researchers then transplanted human melanoma cells into mice, and infected the mice with the various VSV strains. Again, the VSV was able to selectively kill the melanoma cells, but not healthy melanocytes in the mice. Taken together, these results indicate that a virus which is able to quickly kill cells, such as VSV, may be able to selectively target and kill cancerous cells, while avoiding healthy cells.
When the researchers treated the melanoma cells with interferon, a protein produced by cells to help protect the cell from viral infection, both the infectivity and the ability of VSV to kill melanoma cells in vitro was decreased. This indicates that the antiviral immune response generated in normal, noncancerous cells, does not function as efficiently in cancerous melanoma cells. If the cancerous cells are not able to produce innate immune molecules, such as interferon, they cannot stop the viral infection and can be easily killed. Because the cancer cells are unable to protect themselves, the melanoma cells become susceptible to killing by VSV. Adding interferon to the melanoma cells allowed the cells to develop an antiviral immune response, thus preventing cellular killing by the virus.
While this study provides valuable proof of concept information that cancerous cells appear to be more susceptible to killing by viral infection, there are still many questions that must be addressed before this information can be translated to humans. For example, a virus would have to be specifically engineered so as not to cause severe illness in cancer patients, who may already be immunocompromised from standard chemotherapies. In addition, the virus would have to be able to target the tissues in which the cancer is found. Also, many viruses cause the development of a memory immune response, that would result in the virus being quickly destroyed by the host. The immunity of a patient to certain viruses may be unknown, making it difficult to determine if the viral infection would help the patient. Lastly, many of the proteins produced by cells during viral infection can act on neighboring cells. This means that a healthy cell may produce interferon that has a protective effect on nearby cancerous cells. This would prevent the virus from efficiently killing the viral cells.
The study presented above is reminiscent of a study done in the late 1800s, in which a physician injected cancer patients with infectious bacteria. The bacteria caused the host to mount an immune response, producing a protein termed Tumor Necrosis Factor (TNF). Unfortunately, many of the patients succumbed to the infection, and this type of immunotherapy did therefore not become popular for treating cancer patients. However, with more sophisticated knowledge of the immune system and how a virus can affect the host, this type of therapy may soon be another option for cancer patients.
References:
https://www.asm.org/index.php/asm-newsro...rmal-cells
http://www.asm.org/images/Communications...lanoma.pdf
Using attenuated viruses to enhance the effects of chemotherapy drugs has been studied in the past. The virus helps the chemotherapy work more effectively against the cancer cell by weakening the cell so it is more easily killed by the treatment. Adenovirus, which is a causative agent of the common cold, has been studied in many forms for treating cancer. Some genetically engineered strains of adenovirus have been used to infect cancer cells, thereby making them more susceptible to the chemotherapeutics. In addition, adenovirus has been used as a vector for gene therapy, delivering genes to cancer cells that can help remove the cancer from the patient.
Cancer cells tend have deficiencies in the innate immune response that helps protect them from viral infection. This makes cancer cells more susceptible to viral infection than normal, noncancerous cells. Knowing this, researchers wanted to determine if a fast acting virus could selectively infect and kill cancer cells, without harming normal cells. The researcher team first infected melanoma cancer cells in vitro with several strains of a virus called Vesicular Stomatits Virus (VSV). The melanoma cells were efficiently infected and killed by most strains of the virus studied, some of which were able to kill nearly 100% of the melanoma cells. In contrast, healthy melanocytes were not infected as efficiently by VSV. The researchers then transplanted human melanoma cells into mice, and infected the mice with the various VSV strains. Again, the VSV was able to selectively kill the melanoma cells, but not healthy melanocytes in the mice. Taken together, these results indicate that a virus which is able to quickly kill cells, such as VSV, may be able to selectively target and kill cancerous cells, while avoiding healthy cells.
When the researchers treated the melanoma cells with interferon, a protein produced by cells to help protect the cell from viral infection, both the infectivity and the ability of VSV to kill melanoma cells in vitro was decreased. This indicates that the antiviral immune response generated in normal, noncancerous cells, does not function as efficiently in cancerous melanoma cells. If the cancerous cells are not able to produce innate immune molecules, such as interferon, they cannot stop the viral infection and can be easily killed. Because the cancer cells are unable to protect themselves, the melanoma cells become susceptible to killing by VSV. Adding interferon to the melanoma cells allowed the cells to develop an antiviral immune response, thus preventing cellular killing by the virus.
While this study provides valuable proof of concept information that cancerous cells appear to be more susceptible to killing by viral infection, there are still many questions that must be addressed before this information can be translated to humans. For example, a virus would have to be specifically engineered so as not to cause severe illness in cancer patients, who may already be immunocompromised from standard chemotherapies. In addition, the virus would have to be able to target the tissues in which the cancer is found. Also, many viruses cause the development of a memory immune response, that would result in the virus being quickly destroyed by the host. The immunity of a patient to certain viruses may be unknown, making it difficult to determine if the viral infection would help the patient. Lastly, many of the proteins produced by cells during viral infection can act on neighboring cells. This means that a healthy cell may produce interferon that has a protective effect on nearby cancerous cells. This would prevent the virus from efficiently killing the viral cells.
The study presented above is reminiscent of a study done in the late 1800s, in which a physician injected cancer patients with infectious bacteria. The bacteria caused the host to mount an immune response, producing a protein termed Tumor Necrosis Factor (TNF). Unfortunately, many of the patients succumbed to the infection, and this type of immunotherapy did therefore not become popular for treating cancer patients. However, with more sophisticated knowledge of the immune system and how a virus can affect the host, this type of therapy may soon be another option for cancer patients.
References:
https://www.asm.org/index.php/asm-newsro...rmal-cells
http://www.asm.org/images/Communications...lanoma.pdf
by ExpertScie at 04-23-2013, 04:26 PM
0 comments
The way we had developed ourselves during the course of evolution is simply mind-blowing, and the only distinct thing that we had as compared to other (Plants, animals, bacteria, virus etc) is the "passion to explore and innovate" ! Below are few such recent examples :
1. Decay-Fighting Microbes
The Lactic acid production by bacteria living on teeth causes erodes enamel and tooth decay. One of the company in Florida- has engineered a new bacterial strain, called SMaRT, that cannot produce lactic acid. At the same time, it releases an antibiotic that kills the natural decay-causing strain. Thus it now only maintains the teeth but also fight with other bacteria which are bad for teeth. This way, Dentists will only need to swab SMaRT to get rid of decay. Presently, clinical trials, are going on and are at the stage in which once such treatment is given, the tooth remains healthy for life time.
2. Artificial Lymph Nodes
Scientists from Japan's have developed artificial lymph nodes. These organs are very important in immunity as they produce immune cells for fighting infections. The development of artificial Lymph Nodes will be used to replace diseased nodes and also can treat certain diseases which are not easily cured like HIV or Cancer. Not only this but such lymph nodes can be used to boost the immune system and can improve health of patients.
3. Asthma Sensor
Any Asthma attack can lead to serious illness and even death of patients. In such condition the most important thing is timely trigger for treatment. This is emergency situation and all things should be set to recover from it. But now the risk of such sudden asthma attacks is almost irradiated with the development of new sensor called as “Asthama sensor”. This sensor is developed by University of Pittsburgh which is a hand held device, in which, a polymer coated carbon nanotubes that is 100,000 times thinner than human hair, analyzes breath for minute amount of nitric oxide. This gas is produces by lungs prior to asthma attacks. Therefore such sensors will not only indicate any possible attack in patients but also will help them to cure on time in such situations by advance indicative attack.
4. Cancer Spit Test
For any cancer test, now forget biopsies. A new research at University of California –Los Angeles had developed a device that detects oral cancer just by a single drop of saliva. This is based on the proteins that are associated with cancer cells and these proteins react with dyes on the sensor, emitting fluorescent light. This light can be detected under microscope. Not only this, but such technique can also be used for diagnosis of many other diseases by use of saliva as patient’s sample.
5. Biological Pacemaker
Electronic pacemakers save lives, but it has also been observed that such use hardware eventually wears out. Therefore the use of battery fewer alternatives have came in light. Now, researchers at several universities are developing pacemakers by gene expression in stem cells that are injected into damaged regions of the heart. This eliminates the complexity of Biological pacemakers and serves the purpose in more safe way. These pacemakers had brought slow canine hearts back up without complications.
6. Smart Contact Lens
The second-leading cause of blindness is Glaucoma. This is developed when pressure builds inside the eye and damages retinal cells. Contact lenses developed at the University of California-Davis contain conductive wires that continuously monitor pressure and fluid flow within the eyes of at-risk people. The lenses then relay information to a small device worn by the patient; the device wirelessly transmits it to a computer. This constant data flow helps doctors to better understand the causes on time of the disease. Also the lenses automatically dispense drugs in response to pressure changes, thus maintaining its efficiency and intent.
7. Speech Restorer
A new "phonetic speech engine" provides and audible voice for people who have lost the ability to talk. This is developed in conjunction with Texas Instruments, the “Audeo” uses electrodes to detect neuronal signals traveling from the brain to the vocal cords and this way improves the ability to restore it in timely manner. Patients imagine slowly sounding out words; then the quarter-size device which is located in a neck brace wire-less transmits those impulses to a computer, which produces speech.
9. Absorbable Heart Stent
The coronary artery diseases cause blockage and narrowing of arteries. In such condition, today, stents are used to open arteries that have become narrowed. Also with such stents, drug-eluting are combined with release medication that keeps the artery from narrowing again. Now one step further to this, some stents are developed, example the bio-absorbable version made by Abbott Laboratories in Illinois. Unlike metal stents, it does its job and disappears. After six months the stent begins to dissolve that is it is absorbed within the body without causing any side effects. With the time, approximately two years it's completely gone, leaving behind a healthy artery.
10. Muscle Simulator
In cases were bones are broken, treatments are given to join them. During this time nearby muscles often atrophy from lack of use. One of the companies had solved this problem with a battery operated device that develops electrical simulators—small enough to be worn underneath casts that is to exercise muscles and keep them strong during recovery.
Such innovations are mind blowing and this is the way how humans were distinct from others, even during the course of evolution. May it be evolution from primates to modern man or may it be a step of it that is from non-absorbable stent to absorbable stent, the useful difference exists; leading to creativity and well-being!
Please read the full article located here: http://www.popularmechanics.com/science/...hs/4303407
1. Decay-Fighting Microbes
The Lactic acid production by bacteria living on teeth causes erodes enamel and tooth decay. One of the company in Florida- has engineered a new bacterial strain, called SMaRT, that cannot produce lactic acid. At the same time, it releases an antibiotic that kills the natural decay-causing strain. Thus it now only maintains the teeth but also fight with other bacteria which are bad for teeth. This way, Dentists will only need to swab SMaRT to get rid of decay. Presently, clinical trials, are going on and are at the stage in which once such treatment is given, the tooth remains healthy for life time.
2. Artificial Lymph Nodes
Scientists from Japan's have developed artificial lymph nodes. These organs are very important in immunity as they produce immune cells for fighting infections. The development of artificial Lymph Nodes will be used to replace diseased nodes and also can treat certain diseases which are not easily cured like HIV or Cancer. Not only this but such lymph nodes can be used to boost the immune system and can improve health of patients.
3. Asthma Sensor
Any Asthma attack can lead to serious illness and even death of patients. In such condition the most important thing is timely trigger for treatment. This is emergency situation and all things should be set to recover from it. But now the risk of such sudden asthma attacks is almost irradiated with the development of new sensor called as “Asthama sensor”. This sensor is developed by University of Pittsburgh which is a hand held device, in which, a polymer coated carbon nanotubes that is 100,000 times thinner than human hair, analyzes breath for minute amount of nitric oxide. This gas is produces by lungs prior to asthma attacks. Therefore such sensors will not only indicate any possible attack in patients but also will help them to cure on time in such situations by advance indicative attack.
4. Cancer Spit Test
For any cancer test, now forget biopsies. A new research at University of California –Los Angeles had developed a device that detects oral cancer just by a single drop of saliva. This is based on the proteins that are associated with cancer cells and these proteins react with dyes on the sensor, emitting fluorescent light. This light can be detected under microscope. Not only this, but such technique can also be used for diagnosis of many other diseases by use of saliva as patient’s sample.
5. Biological Pacemaker
Electronic pacemakers save lives, but it has also been observed that such use hardware eventually wears out. Therefore the use of battery fewer alternatives have came in light. Now, researchers at several universities are developing pacemakers by gene expression in stem cells that are injected into damaged regions of the heart. This eliminates the complexity of Biological pacemakers and serves the purpose in more safe way. These pacemakers had brought slow canine hearts back up without complications.
6. Smart Contact Lens
The second-leading cause of blindness is Glaucoma. This is developed when pressure builds inside the eye and damages retinal cells. Contact lenses developed at the University of California-Davis contain conductive wires that continuously monitor pressure and fluid flow within the eyes of at-risk people. The lenses then relay information to a small device worn by the patient; the device wirelessly transmits it to a computer. This constant data flow helps doctors to better understand the causes on time of the disease. Also the lenses automatically dispense drugs in response to pressure changes, thus maintaining its efficiency and intent.
7. Speech Restorer
A new "phonetic speech engine" provides and audible voice for people who have lost the ability to talk. This is developed in conjunction with Texas Instruments, the “Audeo” uses electrodes to detect neuronal signals traveling from the brain to the vocal cords and this way improves the ability to restore it in timely manner. Patients imagine slowly sounding out words; then the quarter-size device which is located in a neck brace wire-less transmits those impulses to a computer, which produces speech.
9. Absorbable Heart Stent
The coronary artery diseases cause blockage and narrowing of arteries. In such condition, today, stents are used to open arteries that have become narrowed. Also with such stents, drug-eluting are combined with release medication that keeps the artery from narrowing again. Now one step further to this, some stents are developed, example the bio-absorbable version made by Abbott Laboratories in Illinois. Unlike metal stents, it does its job and disappears. After six months the stent begins to dissolve that is it is absorbed within the body without causing any side effects. With the time, approximately two years it's completely gone, leaving behind a healthy artery.
10. Muscle Simulator
In cases were bones are broken, treatments are given to join them. During this time nearby muscles often atrophy from lack of use. One of the companies had solved this problem with a battery operated device that develops electrical simulators—small enough to be worn underneath casts that is to exercise muscles and keep them strong during recovery.
Such innovations are mind blowing and this is the way how humans were distinct from others, even during the course of evolution. May it be evolution from primates to modern man or may it be a step of it that is from non-absorbable stent to absorbable stent, the useful difference exists; leading to creativity and well-being!
Please read the full article located here: http://www.popularmechanics.com/science/...hs/4303407
by bridgettpayseur at 04-23-2013, 01:41 AM
1 comments
Human immunodeficiency virus (HIV) is the causative agent of acquired immunodeficiency syndrome (AIDS). HIV infects cells that are part of the immune system. The virus binds to CD4, a protein found on the surface of macrophages and helper T cells. Macrophages assist the immune system by ingesting foreign invaders and presenting antigen to the adaptive immune system. When a macrophage takes up an HIV particle, it can become infected, and pass the virus along to CD4+ T cells. Once HIV has infected a cell, its RNA genome is reverse transcribed into DNA. The DNA is then inserted into the host cell’s genome. This means that every time the host cell replicates, the viral genome is also replicated. While integrated in the host cell’s genome, the viral DNA is also transcribed into messenger RNA (mRNA) and translated into protein. This is how new viral particles are produced. The newly made viruses are then released from the host cell, without killing it, to infect new cells.
Antiretroviral is able to decrease HIV to undetectable levels in the bloodstream. However, once antiretroviral therapy is stopped, many patients experience a resurgence of viral load. Because the viral genome is integrated into the host cell’s DNA, it becomes a permanent part of the cell, unless some factor causes it to leave the genome. This means that even if viral RNA is not detectable in a patient’s blood, the patient is likely still infected. The viral genome integrated in the host cell DNA acts as a hidden reservoir for the virus. If the cell is not producing HIV proteins, it will not be targeted by the immune system for destruction, allowing the virus to live on indefinitely in the host. In addition, because antiretroviral therapies block replication of the actual virus, they will not be able to remove the integrated viral genome form cellular DNA.
Scientists had previously discovered a protein found in cells called SAMHD-1 that is able to prevent HIV replication. Strangely, though, the protein did not seem to function in macrophages, which can act as a reservoir for HIV. Researchers from the University of Rochester School of Medicine and Dentistry in Rochester, New York, recently used mass spectrometry to determine if different variations existed of the SAMHD-1 protein. Indeed, they found that SAMHD-1 can either be phosphorylated or unphosphorylated. The phosphorylated version of SAMHD-1 is present when the immune cell is replicating. This state also permits HIV replication, as would be expected. Actively replicating immune cells allow more efficient replication of HIV particles. The unphosphorylated form of SAMHD-1 is present in non-replicating immune cells. This state of SAMHD-1 seems to block HIV replication.
The researchers propose that preventing phosphorylation of SAMHD-1 would prevent HIV replication, and prevent viral reservoirs from being reactivated and repopulating the patient. If therapy preventing SAMHD-1 phosphorylation was administered early during infection for a short time, it might be able to prevent HIV from forming reservoirs in macrophages. This would be a method to prevent the patient from developing chronic HIV infection. However, long term blockade of SAMHD-1 may be problematic for HIV patients. If SAMHD-1 is indeed involved in allowing immune cells to replicate, then preventing the protein from functioning would also prevent strong immune responses from developing. This would leave the patient in an immunocompromised state, which is the end result of HIV infection.
The balance of immune activation during HIV infection has indeed been problematic for researchers trying to develop treatments and vaccines for HIV. It has long been known that active, replicating CD4+ T cells are more efficient at promoting replication of HIV. Many studies have shown that immune activation, particularly during the early stages of HIV infection in humans or SIV infection in monkeys, can cause extremely high viral loads and more severe disease. However, preventing an immune response during HIV infection may also result in increased viral loads. A strong T cell immune response is necessary to control viral levels. CD8+ T cells, which are able to directly kill HIV-infected CD4+ T cells, are an important factor in HIV immunity. In order to be properly activated, CD8+ T cells require antigen from HIV to be presented by macrophages, which are a target of HIV infection. Preventing the establishment of HIV infection, and curing chronic HIV infection, is therefore a delicate balancing act of the immune system that scientists still do not fully understand.
References:
http://www.sciencedaily.com/releases/201...164630.htm
Antiretroviral is able to decrease HIV to undetectable levels in the bloodstream. However, once antiretroviral therapy is stopped, many patients experience a resurgence of viral load. Because the viral genome is integrated into the host cell’s DNA, it becomes a permanent part of the cell, unless some factor causes it to leave the genome. This means that even if viral RNA is not detectable in a patient’s blood, the patient is likely still infected. The viral genome integrated in the host cell DNA acts as a hidden reservoir for the virus. If the cell is not producing HIV proteins, it will not be targeted by the immune system for destruction, allowing the virus to live on indefinitely in the host. In addition, because antiretroviral therapies block replication of the actual virus, they will not be able to remove the integrated viral genome form cellular DNA.
Scientists had previously discovered a protein found in cells called SAMHD-1 that is able to prevent HIV replication. Strangely, though, the protein did not seem to function in macrophages, which can act as a reservoir for HIV. Researchers from the University of Rochester School of Medicine and Dentistry in Rochester, New York, recently used mass spectrometry to determine if different variations existed of the SAMHD-1 protein. Indeed, they found that SAMHD-1 can either be phosphorylated or unphosphorylated. The phosphorylated version of SAMHD-1 is present when the immune cell is replicating. This state also permits HIV replication, as would be expected. Actively replicating immune cells allow more efficient replication of HIV particles. The unphosphorylated form of SAMHD-1 is present in non-replicating immune cells. This state of SAMHD-1 seems to block HIV replication.
The researchers propose that preventing phosphorylation of SAMHD-1 would prevent HIV replication, and prevent viral reservoirs from being reactivated and repopulating the patient. If therapy preventing SAMHD-1 phosphorylation was administered early during infection for a short time, it might be able to prevent HIV from forming reservoirs in macrophages. This would be a method to prevent the patient from developing chronic HIV infection. However, long term blockade of SAMHD-1 may be problematic for HIV patients. If SAMHD-1 is indeed involved in allowing immune cells to replicate, then preventing the protein from functioning would also prevent strong immune responses from developing. This would leave the patient in an immunocompromised state, which is the end result of HIV infection.
The balance of immune activation during HIV infection has indeed been problematic for researchers trying to develop treatments and vaccines for HIV. It has long been known that active, replicating CD4+ T cells are more efficient at promoting replication of HIV. Many studies have shown that immune activation, particularly during the early stages of HIV infection in humans or SIV infection in monkeys, can cause extremely high viral loads and more severe disease. However, preventing an immune response during HIV infection may also result in increased viral loads. A strong T cell immune response is necessary to control viral levels. CD8+ T cells, which are able to directly kill HIV-infected CD4+ T cells, are an important factor in HIV immunity. In order to be properly activated, CD8+ T cells require antigen from HIV to be presented by macrophages, which are a target of HIV infection. Preventing the establishment of HIV infection, and curing chronic HIV infection, is therefore a delicate balancing act of the immune system that scientists still do not fully understand.
References:
http://www.sciencedaily.com/releases/201...164630.htm
by bridgettpayseur at 04-22-2013, 11:42 AM
1 comments
The ability of certain populations to evade infection is not a new concept in science. For example, people of West African descent, an area where malaria is highly prevelant, have some degree of protection against malaria. This is because the sickle cell allele alters the shape and function of the hemoglobin protein enough to prevent the malaria parasite from entering and infecting the host cell. People with one copy of the sickle cell allele have a natural degree of protection against malaria. People with two copies of the sickle cell allele, however, are affected with sickle cell anemia, a painful, debilitating condition in which the red blood cells are misshapen. The sickle cell allele remains within the population to provide protection against malaria.
Another example is the mutation of a co-receptor protein used by human immunodeficiency virus (HIV) that is found in European populations. HIV requires a main receptor on the cell, called CD4, to infect T cells. It also requires an additional co-receptor. Some strains of HIV utilize a co-receptor called CXCR4, and others utilize a co-receptor called CCR5. A mutation in the CCR5 gene, which is most commonly seen in European populations, prevents CCR5-tropic strains of HIV from entering the cell. This is because the virus is unable to properly bind to and recognize the cell. Individuals who have the CCR5 mutation are considered immune to these specific strains of HIV. In fact, an HIV patient who was treated with a bone marrow transplant from a donor with this CCR5 mutation was able to clear his HIV infection, and has not had detectable virus levels since the transplant.
These mutations likely occurred to help protect at-risk populations from infectious diseases. They are a functional way to prevent infection- they rely on changing the protein so that the infectious agent cannot gain entry to the cell. These mutations do not have an effect on the actual immune response developed in response to the infection. Rather, they help prevent the infection from being established in the first place.
The difference in a person’s ability to fight infection based on ethnicity has only recently been studied. Researchers from Simon Fraser University in British Columbia, Canada, sequenced the immunoglobulin heavy chain (IGH) gene in 425 volunteers of different ethnic origins. The IGH gene codes for a part of the protein that makes up most of the antibodies produced by humans. It is about 1 million nucleotides long, and consists of many segments that are rearranged in the B cell to produce unique antibodies. The researchers found astounding differences in the composition of the IGH gene based on ethnic origin in the volunteers after sequencing the genes. Some ethnicities seemed to have large deletions of segments of the IGH gene, as well as large insertions. The different composition of the IGH gene would result in a different array of antibodies being produced between the ethnic groups tested.
The researchers hypothesized that people are subjected to different types of pathogens in different parts of the world. Over time, portions of the IGH gene were either deleted or inserted in order to help the individuals produce antibodies needed against these pathogens. If one type of bacterium, for example, was not often seen in parts of Asia, the segments of the IGH gene that would develop antibody against that bacterium would eventually be lost from the gene. If this bacterium was a prominent threat to people in Europe, however, it would make sense that the IGH locus would maintain that portion of the gene in order to be able to produce the proper antibody.
The study results have implications for both treatments and vaccinations against infectious disease. Certain populations may not be able to respond with an effective immune response against a specific pathogen, meaning that alternative forms of treatment would be required to help remove the infection. In addition, if someone is unable to properly respond to a vaccination because he or she is unable to produce the desired antibody, the vaccine would be ineffective. This could prevent large areas of the world from benefiting from potentially life-saving vaccines. The research indicates that universal treatments and vaccines should be properly evaluated in diverse populations to ensure that everyone is able to benefit.
References:
http://www.upi.com/Science_News/2013/04/...366414892/
Another example is the mutation of a co-receptor protein used by human immunodeficiency virus (HIV) that is found in European populations. HIV requires a main receptor on the cell, called CD4, to infect T cells. It also requires an additional co-receptor. Some strains of HIV utilize a co-receptor called CXCR4, and others utilize a co-receptor called CCR5. A mutation in the CCR5 gene, which is most commonly seen in European populations, prevents CCR5-tropic strains of HIV from entering the cell. This is because the virus is unable to properly bind to and recognize the cell. Individuals who have the CCR5 mutation are considered immune to these specific strains of HIV. In fact, an HIV patient who was treated with a bone marrow transplant from a donor with this CCR5 mutation was able to clear his HIV infection, and has not had detectable virus levels since the transplant.
These mutations likely occurred to help protect at-risk populations from infectious diseases. They are a functional way to prevent infection- they rely on changing the protein so that the infectious agent cannot gain entry to the cell. These mutations do not have an effect on the actual immune response developed in response to the infection. Rather, they help prevent the infection from being established in the first place.
The difference in a person’s ability to fight infection based on ethnicity has only recently been studied. Researchers from Simon Fraser University in British Columbia, Canada, sequenced the immunoglobulin heavy chain (IGH) gene in 425 volunteers of different ethnic origins. The IGH gene codes for a part of the protein that makes up most of the antibodies produced by humans. It is about 1 million nucleotides long, and consists of many segments that are rearranged in the B cell to produce unique antibodies. The researchers found astounding differences in the composition of the IGH gene based on ethnic origin in the volunteers after sequencing the genes. Some ethnicities seemed to have large deletions of segments of the IGH gene, as well as large insertions. The different composition of the IGH gene would result in a different array of antibodies being produced between the ethnic groups tested.
The researchers hypothesized that people are subjected to different types of pathogens in different parts of the world. Over time, portions of the IGH gene were either deleted or inserted in order to help the individuals produce antibodies needed against these pathogens. If one type of bacterium, for example, was not often seen in parts of Asia, the segments of the IGH gene that would develop antibody against that bacterium would eventually be lost from the gene. If this bacterium was a prominent threat to people in Europe, however, it would make sense that the IGH locus would maintain that portion of the gene in order to be able to produce the proper antibody.
The study results have implications for both treatments and vaccinations against infectious disease. Certain populations may not be able to respond with an effective immune response against a specific pathogen, meaning that alternative forms of treatment would be required to help remove the infection. In addition, if someone is unable to properly respond to a vaccination because he or she is unable to produce the desired antibody, the vaccine would be ineffective. This could prevent large areas of the world from benefiting from potentially life-saving vaccines. The research indicates that universal treatments and vaccines should be properly evaluated in diverse populations to ensure that everyone is able to benefit.
References:
http://www.upi.com/Science_News/2013/04/...366414892/
by Nikolas at 04-21-2013, 11:28 AM
0 comments
Cells previously known to create inflammation and generate fat have been discovered to be crucial in regenerating muscle tissue.
Scientists at UC San Francisco have performed the study. The experiments were performed on mice, and the results showed that an immune cell, which has been already substantially studies, called the eosinophil, has two more previously unknown beneficial roles. By clearing out cellular debris from damaged tissue it allows the new cells to re-grow and removes the threat from infection, and by teaming up with a type of cell that can make fat to instead trigger muscle regrowth it presents a key step in muscle regeneration.
The study was led by Ajay Chawla, MD, PhD, an associate professor of medicine at the UCSF Cardiovascular Research Institute. It showed that after eosinophils migrate to the site of injury, they work together with a progenitor cell, immature cells similar to stem cells, to power the formation of new muscle fibers. The progenitors are called the fibro/adipogenic cells (FAP), and they do not spin off muscle cells directly, instead serving as another key step in the activation of regeneration.
"Without eosinophils you cannot regenerate muscle," Chawla said.
FAP cells have previously been studied and are known for their roles in making fat, which occurs naturally as the body ages or experiences prolonged immobility. They also have been shown to make cells that form connective tissue. But the new study showed that FAP cells can also team up with eosinophils to make injured muscles regenerate and strengthen, rather than make them fatter, at least in mice.
In a cellular chain reaction, the team found that when eosinophils are at the site of muscle injury they secrete a molecule called IL-4, and the fibro/adipogenic progenitor cells respond by iniciating mitosis, increasing their numbers. And instead of becoming fat cells, as they were expected to, they react with the true muscle stem cells and trigger the regrowth and regeneration of muscle fibers.
"They wake up the cells in muscle that divide and form muscle fibers," Chawla stated.
Eosinophils normally help fight off bacteria and parasites, as do other immune cells, expecially of this subgroup, but eosinophils are more often considered in the maladaptive roles they have in allergies and other inflammatory reactions. Eosinophils comprise only a few percent of immune cells, being a relatively smaller subgroup compared to basophiles or neutrophiles.
The research team found that, even before active muscle repair incurs, the chain reaction initiated by eosinophils performs another necessary task, that is cleaning out the injury site of any debris or cellular garbage.
"Eosinophils, acting via FAPs, are needed for the rapid clearance of necrotic debris, a process that is necessary for timely and complete regeneration of tissues," Chawla said.
It has been thought until now that bigger and more common immune cells called macrophages, which are known to have large appetites and a propensity to ‘eat up’ debris in other injury or cell death scenarios are the ones tasked with cleaning up messes and remains within distressed or injured muscle tissue.
"Bites from venomous animals, many toxicants, and parasitic worms all trigger somewhat similar immune responses that cause injury. We want to know if eosinophils and FAPs are universally employed in these situations as a way to get rid of debris without triggering severe reactions such as anaphylactic shock." – said Chawla.
Other researchers working on this project are: postdoctoral fellow Jose E. Heredia, PhD; specialist Lata Mukundan, PhD; technician Francis Chen; Rahul Deo, MD, PhD, an assistant professor of medicine in residence; and Richard M. Locksley, MD, an immunologist and professor of medicine, Stanford researchers Thomas Rando, MD, PhD, and graduate student Alisa Mueller. The National Institutes for Health and the California Institute for Regenerative Medicine were the main founders of this research
Resources:
Jose E. Heredia, Lata Mukundan, Francis M. Chen, Alisa A. Mueller, Rahul C. Deo, Richard M. Locksley, Thomas A. Rando, Ajay Chawla. Type 2 Innate Signals Stimulate Fibro/Adipogenic Progenitors to Facilitate Muscle Regeneration. Cell, 2013
Scientists at UC San Francisco have performed the study. The experiments were performed on mice, and the results showed that an immune cell, which has been already substantially studies, called the eosinophil, has two more previously unknown beneficial roles. By clearing out cellular debris from damaged tissue it allows the new cells to re-grow and removes the threat from infection, and by teaming up with a type of cell that can make fat to instead trigger muscle regrowth it presents a key step in muscle regeneration.
The study was led by Ajay Chawla, MD, PhD, an associate professor of medicine at the UCSF Cardiovascular Research Institute. It showed that after eosinophils migrate to the site of injury, they work together with a progenitor cell, immature cells similar to stem cells, to power the formation of new muscle fibers. The progenitors are called the fibro/adipogenic cells (FAP), and they do not spin off muscle cells directly, instead serving as another key step in the activation of regeneration.
"Without eosinophils you cannot regenerate muscle," Chawla said.
FAP cells have previously been studied and are known for their roles in making fat, which occurs naturally as the body ages or experiences prolonged immobility. They also have been shown to make cells that form connective tissue. But the new study showed that FAP cells can also team up with eosinophils to make injured muscles regenerate and strengthen, rather than make them fatter, at least in mice.
In a cellular chain reaction, the team found that when eosinophils are at the site of muscle injury they secrete a molecule called IL-4, and the fibro/adipogenic progenitor cells respond by iniciating mitosis, increasing their numbers. And instead of becoming fat cells, as they were expected to, they react with the true muscle stem cells and trigger the regrowth and regeneration of muscle fibers.
"They wake up the cells in muscle that divide and form muscle fibers," Chawla stated.
Eosinophils normally help fight off bacteria and parasites, as do other immune cells, expecially of this subgroup, but eosinophils are more often considered in the maladaptive roles they have in allergies and other inflammatory reactions. Eosinophils comprise only a few percent of immune cells, being a relatively smaller subgroup compared to basophiles or neutrophiles.
The research team found that, even before active muscle repair incurs, the chain reaction initiated by eosinophils performs another necessary task, that is cleaning out the injury site of any debris or cellular garbage.
"Eosinophils, acting via FAPs, are needed for the rapid clearance of necrotic debris, a process that is necessary for timely and complete regeneration of tissues," Chawla said.
It has been thought until now that bigger and more common immune cells called macrophages, which are known to have large appetites and a propensity to ‘eat up’ debris in other injury or cell death scenarios are the ones tasked with cleaning up messes and remains within distressed or injured muscle tissue.
"Bites from venomous animals, many toxicants, and parasitic worms all trigger somewhat similar immune responses that cause injury. We want to know if eosinophils and FAPs are universally employed in these situations as a way to get rid of debris without triggering severe reactions such as anaphylactic shock." – said Chawla.
Other researchers working on this project are: postdoctoral fellow Jose E. Heredia, PhD; specialist Lata Mukundan, PhD; technician Francis Chen; Rahul Deo, MD, PhD, an assistant professor of medicine in residence; and Richard M. Locksley, MD, an immunologist and professor of medicine, Stanford researchers Thomas Rando, MD, PhD, and graduate student Alisa Mueller. The National Institutes for Health and the California Institute for Regenerative Medicine were the main founders of this research
Resources:
Jose E. Heredia, Lata Mukundan, Francis M. Chen, Alisa A. Mueller, Rahul C. Deo, Richard M. Locksley, Thomas A. Rando, Ajay Chawla. Type 2 Innate Signals Stimulate Fibro/Adipogenic Progenitors to Facilitate Muscle Regeneration. Cell, 2013
by sale0303 at 04-21-2013, 01:59 AM
1 comments
Multiple sclerosis (MS) is chronic demyelinating, inflammatory autoimmune disease of the central nervous system. This disease gives permanent lesion to the central nervous system. This autoimmune disease attacks parts of myelin sheath. This myelin sheath has role in insulation of neural communication. Destruction of oligodendrocytes is damaging the communication between two cells, because oligodendrocytes receive impulses via axon of another neural cell. Primary etiology of MS remains unknown and it is possible that it has more than one cause. This disease affects women two times more than men, but men have more aggressive types of MS. It is followed by unpredictable periods of remissions and relapses. After certain number of relapses and remissions, patients accumulate neural lesions, and it leads to disability.
Current Treatment of Multiple Sclerosis
Modern medicine has no cure for multiple sclerosis. Treatments are based on reducing the progress of the disease and management of the symptoms. Due to severity of the symptoms, in some cases there is no need for any kind of treatment.
There are three types of strategies for treatment of multiple sclerosis- treatment of attacks, slowing down of the progress and treatment of symptoms. Due to stage of disease, various drugs are used. For acute attacks glucocorticoids are used. Beta- interferons are used in modification of the multiple sclerosis course, and potassium blockers and oral vitamin D are used in treatment of the symptoms.
New Treatments of Multiple Sclerosis
There were many researches on mice, and they had encouraging results. First problem for scientist studies on mice was to find neural lesion similar to lesion in multiple sclerosis in human CNS. This problem was solved because they found out that lesions experimental autoimmune encephalomyelitis is identical to MS lesions in human CNS. These lesion were treated with stem cells, and they gave extraordinary results. Infiltrated stem cells gave gradual improvement in multiple sclerosis symptoms. This was just a beginning in treatment of multiple sclerosis with stem cells.
Therapy of MS with purified stem cells, isolated from bone marrow and umbilical cord blood, is promising way of treatment. These cells have been named CD 34+ cells. The CD 34+ cell can migrate to lesion location and there it can proliferate and differentiate in specific cell which can repair the damage. In case of multiple sclerosis, these cells transform in oligodendrocytes. This way of treatment is now under development, and we expect results as soon as possible.
Use of Various Stem Cells in Treatment of MS
Hematopoietic stem cell have been used in treatment of leukemia and other blood cancers. The bone marrow was transplanted in this process. Fortunately, scientist have discovered that this way of treatment is suitable for patients with very aggressive forms of multiple sclerosis. The procedure consists of destruction of patients immune system which has immune memory. When bone marrow is destructed, patient receives previously taken hematopoietic stem cells from himself, or from some other donor. Transplanted hematopoietic stem cells have no immune memory. Therefore, they should not have tendency of autoimmune destruction. However, this procedure is not used in treatment of every single patient with multiple sclerosis, because destruction of immune system carries certain dose of risk and possibility of fatal complication.
An ideal resource for treatment of MS with stem cells were neural stem cells. These cells were considered as very good way of filling destroyed loci with oligodendrocytes. This research showed a lot of promise, but it didn’t show expected results. Results were disappointing because, this way of treatment showed low level of oligodendrocytes renewal.
Future Possibilities of MS Treatment
Another type of cells are being tested, like precursors of oligodendrocytes. Application of cell cultures from laboratories is one of the strategies, but cells cultures should be placed in multiple regions of brain, and it is not easy goal to achieve. Another strategy is stimulation of remained brain oligodendrocyte precursors. These precursors would be transformed in oligodendrocytes and then these oligodendrocytes would migrate to demyelination loci and repair demyelinated neurons.
The Latest Discoveries in Stem Cell Treatment of MS
Scientists have discovered procedure which can convert human skin cells to neural cells. This revolutionary discovery can replace neurons in many neurodegenerative diseases like multiple sclerosis, but also in other myelin degenerative processes. In these neurodegenerative conditions myelin cells are destroyed, and they cannot be replaced. However, this newest research gives an opportunity of producing large quantities of myelinating cells which isolate communication between two neural cells. Basically, skin fibroblasts, very common cells in human skin, are converted into oligodendrocytes. This process includes reprogramming of the cell. Scientists have exchanged structure of three protein types, and that induced fibroblast to change into oligodendrocyte precursors.
Research team developed billions of induced oligodendrocytes progenitor cells in short time, and, more important thing, they have showed that these cells gave significantly improvement in reparation of oligodendrocytes in mice.
In past, oligodendrocytes progenitor cells were produced only from embryonic stem cells, and this method had some limitations. The main limitation was disability to produce quick and stabile amount of oligodendrocytes, but with new method this difficulty became past. If this method shows good results on human trials, it could be common treatment for many people with myelin disorders.
Another recent study focuses on glial progenitor cells. These cells can differentiate in astrocytes and oligodendrocytes. However, this progenitor cells stop dividing themselves or even they differentiate into specialized cells, and this is the main obstacle in further research. Scientist have found out that the main role in cell division plays beta-catenin and it is regulated by glycogen synthase kinase 3 beta(GSK3B). If researchers only could block this synthase (because this GSK3B is being blocked during cell division), they would solve this problem with early differentiation.
Summary
According to these researches, MS could be stopped or even cured if at least one of these researches succeeds in their attention. Stem cells have showed that they present future in modern medicine, and it's all up to scientists to find out the best way of curing these harmful diseases.
Current Treatment of Multiple Sclerosis
Modern medicine has no cure for multiple sclerosis. Treatments are based on reducing the progress of the disease and management of the symptoms. Due to severity of the symptoms, in some cases there is no need for any kind of treatment.
There are three types of strategies for treatment of multiple sclerosis- treatment of attacks, slowing down of the progress and treatment of symptoms. Due to stage of disease, various drugs are used. For acute attacks glucocorticoids are used. Beta- interferons are used in modification of the multiple sclerosis course, and potassium blockers and oral vitamin D are used in treatment of the symptoms.
New Treatments of Multiple Sclerosis
There were many researches on mice, and they had encouraging results. First problem for scientist studies on mice was to find neural lesion similar to lesion in multiple sclerosis in human CNS. This problem was solved because they found out that lesions experimental autoimmune encephalomyelitis is identical to MS lesions in human CNS. These lesion were treated with stem cells, and they gave extraordinary results. Infiltrated stem cells gave gradual improvement in multiple sclerosis symptoms. This was just a beginning in treatment of multiple sclerosis with stem cells.
Therapy of MS with purified stem cells, isolated from bone marrow and umbilical cord blood, is promising way of treatment. These cells have been named CD 34+ cells. The CD 34+ cell can migrate to lesion location and there it can proliferate and differentiate in specific cell which can repair the damage. In case of multiple sclerosis, these cells transform in oligodendrocytes. This way of treatment is now under development, and we expect results as soon as possible.
Use of Various Stem Cells in Treatment of MS
Hematopoietic stem cell have been used in treatment of leukemia and other blood cancers. The bone marrow was transplanted in this process. Fortunately, scientist have discovered that this way of treatment is suitable for patients with very aggressive forms of multiple sclerosis. The procedure consists of destruction of patients immune system which has immune memory. When bone marrow is destructed, patient receives previously taken hematopoietic stem cells from himself, or from some other donor. Transplanted hematopoietic stem cells have no immune memory. Therefore, they should not have tendency of autoimmune destruction. However, this procedure is not used in treatment of every single patient with multiple sclerosis, because destruction of immune system carries certain dose of risk and possibility of fatal complication.
An ideal resource for treatment of MS with stem cells were neural stem cells. These cells were considered as very good way of filling destroyed loci with oligodendrocytes. This research showed a lot of promise, but it didn’t show expected results. Results were disappointing because, this way of treatment showed low level of oligodendrocytes renewal.
Future Possibilities of MS Treatment
Another type of cells are being tested, like precursors of oligodendrocytes. Application of cell cultures from laboratories is one of the strategies, but cells cultures should be placed in multiple regions of brain, and it is not easy goal to achieve. Another strategy is stimulation of remained brain oligodendrocyte precursors. These precursors would be transformed in oligodendrocytes and then these oligodendrocytes would migrate to demyelination loci and repair demyelinated neurons.
The Latest Discoveries in Stem Cell Treatment of MS
Scientists have discovered procedure which can convert human skin cells to neural cells. This revolutionary discovery can replace neurons in many neurodegenerative diseases like multiple sclerosis, but also in other myelin degenerative processes. In these neurodegenerative conditions myelin cells are destroyed, and they cannot be replaced. However, this newest research gives an opportunity of producing large quantities of myelinating cells which isolate communication between two neural cells. Basically, skin fibroblasts, very common cells in human skin, are converted into oligodendrocytes. This process includes reprogramming of the cell. Scientists have exchanged structure of three protein types, and that induced fibroblast to change into oligodendrocyte precursors.
Research team developed billions of induced oligodendrocytes progenitor cells in short time, and, more important thing, they have showed that these cells gave significantly improvement in reparation of oligodendrocytes in mice.
In past, oligodendrocytes progenitor cells were produced only from embryonic stem cells, and this method had some limitations. The main limitation was disability to produce quick and stabile amount of oligodendrocytes, but with new method this difficulty became past. If this method shows good results on human trials, it could be common treatment for many people with myelin disorders.
Another recent study focuses on glial progenitor cells. These cells can differentiate in astrocytes and oligodendrocytes. However, this progenitor cells stop dividing themselves or even they differentiate into specialized cells, and this is the main obstacle in further research. Scientist have found out that the main role in cell division plays beta-catenin and it is regulated by glycogen synthase kinase 3 beta(GSK3B). If researchers only could block this synthase (because this GSK3B is being blocked during cell division), they would solve this problem with early differentiation.
Summary
According to these researches, MS could be stopped or even cured if at least one of these researches succeeds in their attention. Stem cells have showed that they present future in modern medicine, and it's all up to scientists to find out the best way of curing these harmful diseases.
by bridgettpayseur at 04-21-2013, 01:19 AM
0 comments
Interferons are a type of protein associated with the immune response to help protect the host against infection. Generally, Type I Interferons (IFN-I) will help put the cell in an antiviral state, by stalling the cell cycle to prevent viral replication. IFN-I will also alert nearby cells to the presence of virus, and helps prevent the infection from spreading. A strong IFN-I response is normally associated with rapid control of viral infection. The result of IFN-I production by infected cells can lead to activation of the adaptive immune system, including CD8+ cytolytic T cells. CD8+ T cells are rapidly activated during viral infection, and can help clear the infection by killing infected cells.
While many viral infections can often be cleared by the immune system, there are viruses that can remain in the host persistently. These viruses seem to work by changing the proteins that are expressed by the host immune cells. The viruses are able to induce expression of proteins that limit the immune response, such as interleukin-10 (IL-10) and PD-1, a death receptor on the immune cells. This means that immune cells, such as T cells, are not able to function as well against the viral infection, which results in a condition termed exhaustion of the immune system. When the immune system is unable to destroy the virus, the infection becomes persistent.
Recently, however, researchers at the Scripps Research Institute in California demonstrated that IFN-I levels were actually much higher in mice infected with a chronic viral infection, compared to mice infected with an acute viral infection. This occurred as early as one day post infection. The results lead the researchers to speculate that IFN-I played a role in maintaining persistent viral infection. To test this hypothesis, they used antibodies to block IFN-I receptors. The antibodies would prevent the IFN-I from performing its function in the cell. When the receptors were blocked with antibody, the researchers found decreased expression of immune inhibiting proteins, including IL-10 and PD-L1, the ligand for PD-1. The decrease in these inhibitory proteins allowed the adaptive immune system to mount a response, and CD8+ T cells were able to kill the virus-infected cells and clear the infection. The researchers also found that blocking IFN-I receptors in a previously established persistent infection helped remove the signals that cause T cell exhaustion. The T cells were then able to function properly, and again, could clear the infection.
It is not clear how IFN-I assists the virus in establishing chronic infection. As the researchers discovered, IFN-I appears to play a role in increasing production of immune inhibitory cytokines such as IL-10 and PD-1, which prevents T cells from carrying out their proper function. In addition, IFN-I seemed to play a role in causing excessive production of inflammatory cytokines, a condition called cytokine storm. The cytokines may cause inflammation, and may even be responsible to damage to architecture in lymphatic organs such as the spleen. When these organs are damaged, Dendritic Cells (DCs), the innate immune cells that present antigen to and activate T cells, are not able to meet with the T cells. This prevents T cell activation, which would also be a contributing factor to chronic viral infection. When the researchers blocked IFN-I receptors, they also noticed decreased production of these hyperinflammatory cytokines, and the structure of the lymphatic organs was restored.
The researchers hope this information can be used to help treat chronic viral infections in humans, such as HIV; Hepatitis B and Hepatitis C; and Epstein-Barr Virus. They plan to not only study the effects of antibody blockade of IFN-I receptors, but also small pharmacologic molecules. Because there are more than 20 different molecules in the IFN-I family, it may be beneficial to study precisely which molecules are involved in establishing and maintaining viral persistence. This would allow researchers and clinicians to selectively inhibit problematic IFN-I molecules, but still permit helpful IFN-I molecules to function. This could prevent the early increase in viral load detected in the blood after IFN-I receptors were inhibited in mice, and reduce the severity of illness suffered by the patient. By lifting immune restrictions imposed by the chronic viral infection, the adaptive immune response may be able to better fight these infections and clear the virus.
References:
http://www.sciencedaily.com/releases/201...142815.htm
http://pathmicro.med.sc.edu/mhunt/interferon.htm
While many viral infections can often be cleared by the immune system, there are viruses that can remain in the host persistently. These viruses seem to work by changing the proteins that are expressed by the host immune cells. The viruses are able to induce expression of proteins that limit the immune response, such as interleukin-10 (IL-10) and PD-1, a death receptor on the immune cells. This means that immune cells, such as T cells, are not able to function as well against the viral infection, which results in a condition termed exhaustion of the immune system. When the immune system is unable to destroy the virus, the infection becomes persistent.
Recently, however, researchers at the Scripps Research Institute in California demonstrated that IFN-I levels were actually much higher in mice infected with a chronic viral infection, compared to mice infected with an acute viral infection. This occurred as early as one day post infection. The results lead the researchers to speculate that IFN-I played a role in maintaining persistent viral infection. To test this hypothesis, they used antibodies to block IFN-I receptors. The antibodies would prevent the IFN-I from performing its function in the cell. When the receptors were blocked with antibody, the researchers found decreased expression of immune inhibiting proteins, including IL-10 and PD-L1, the ligand for PD-1. The decrease in these inhibitory proteins allowed the adaptive immune system to mount a response, and CD8+ T cells were able to kill the virus-infected cells and clear the infection. The researchers also found that blocking IFN-I receptors in a previously established persistent infection helped remove the signals that cause T cell exhaustion. The T cells were then able to function properly, and again, could clear the infection.
It is not clear how IFN-I assists the virus in establishing chronic infection. As the researchers discovered, IFN-I appears to play a role in increasing production of immune inhibitory cytokines such as IL-10 and PD-1, which prevents T cells from carrying out their proper function. In addition, IFN-I seemed to play a role in causing excessive production of inflammatory cytokines, a condition called cytokine storm. The cytokines may cause inflammation, and may even be responsible to damage to architecture in lymphatic organs such as the spleen. When these organs are damaged, Dendritic Cells (DCs), the innate immune cells that present antigen to and activate T cells, are not able to meet with the T cells. This prevents T cell activation, which would also be a contributing factor to chronic viral infection. When the researchers blocked IFN-I receptors, they also noticed decreased production of these hyperinflammatory cytokines, and the structure of the lymphatic organs was restored.
The researchers hope this information can be used to help treat chronic viral infections in humans, such as HIV; Hepatitis B and Hepatitis C; and Epstein-Barr Virus. They plan to not only study the effects of antibody blockade of IFN-I receptors, but also small pharmacologic molecules. Because there are more than 20 different molecules in the IFN-I family, it may be beneficial to study precisely which molecules are involved in establishing and maintaining viral persistence. This would allow researchers and clinicians to selectively inhibit problematic IFN-I molecules, but still permit helpful IFN-I molecules to function. This could prevent the early increase in viral load detected in the blood after IFN-I receptors were inhibited in mice, and reduce the severity of illness suffered by the patient. By lifting immune restrictions imposed by the chronic viral infection, the adaptive immune response may be able to better fight these infections and clear the virus.
References:
http://www.sciencedaily.com/releases/201...142815.htm
http://pathmicro.med.sc.edu/mhunt/interferon.htm
by bridgettpayseur at 04-21-2013, 12:45 AM
0 comments
Malaria is one of the top three leading causes of death due to infectious disease worldwide. As many as 300 million people are infected with malaria worldwide every year, resulting in an estimated one to three million deaths. Malaria is caused by several parasites of the genus Plasmodium, with P. falciparum being responsible for most of the deaths. While antimalarial therapies are available to help those who are infected, a protective vaccine against malaria is still urgently needed. Because malaria mostly affects people in poverty stricken areas, the vaccine should be cheap and easy to administer. This would help maximize compliance and be best at preventing disease.
Malaria is a protozoan parasite, which means it is caused by a eukaryotic pathogen. Bacteria, which are often used to produce proteins for vaccine constructs, are prokaryotic, and lack much of the complexity of the eukaryotic cell. The bacteria are not able to process proteins in the same fashion that eukaryotes can, making it difficult to develop a properly folded and processed malaria protein in a bacterial vector. If the proteins produced by the bacteria are not identical to the proteins produced by malaria parasites, they will not be recognized by the immune system. This has hampered anti-malaria vaccine production. A eukaryotic vector would be a better candidate, as it would have to proper machinery to process the proteins so that they are identical to the malaria-produced proteins.
A team of researchers at the University of California at San Diego have attempted to make an edible vaccine against malaria. They engineered algae to produce a protein from P. falciparum that has strong immunogenicity in mice. In addition, the researchers engineered the algae to produce a protein from Vibrio cholera, which would be fused to the P. falciparum antigen. The V. cholera protein sticks to intestinal cells. The researchers wanted the engineered fusion protein from the algae to be taken up by the intestinal cells and presented to the immune system within the gastrointestinal tract. When mice were fed the freeze dried algae, they produced substantial amounts of IgA antibody against the P. falciparum protein. IgA is an isotype of antibody that is primarily produced by mucosal immune responses, and is effective against pathogens that infect the mucosae.
Malaria, however, is injected into the blood stream via the bite of a mosquito. The malaria parasite first goes to the liver, where it develops into a form that can then infect red blood cells. The IgA antibody produced by the mice in response to the edible algae vaccine would be ineffective against malaria parasites. IgG antibodies, which are produced in the blood stream, are considered to be more effective against malaria infection by many researchers. Learning how to develop the proper antibody response is one of the important aspects of vaccine production. Improper antibody responses would not be effective at preventing infection.
Even though the vaccine construct does not appear suitable for use against malaria, the researchers are hopeful that it can be used against a variety of mucosal diseases. Many viral and bacterial infections are food borne; they enter the host through the digestive tract. In these cases, a mucosal IgA antibody response produced in the gastrointestinal tract would be very beneficial. The edible algae vaccine vector could be used against such food borne pathogens as Salmonella and E. coli.
While it might seem unusual at first to consider using an oral vaccine, which would target immune cells in the digestive tract, against a pathogen that infects blood cells, the concept is not as far out as one might think. The mucosal immune system and the systemic immune system are connected. Food digested in the gastrointestinal tract is transported to the blood in order to be delivered to other cells in the body. The same is true of antigens. Research has previously demonstrated that certain mucosal vaccination routes, such as a nasal vaccine, can be very effective at eliciting strong systemic immune responses. In fact, the researchers in the above study were able to detect blood IgG antibodies against the V. cholera portion of the fusion protein. This demonstrates clearly that the construct can induce systemic immune responses. The researchers, however, are not sure why the mice did not produce IgG antibodies against the malaria protein.
References:
http://www.sciencedaily.com/releases/201...132607.htm
Malaria is a protozoan parasite, which means it is caused by a eukaryotic pathogen. Bacteria, which are often used to produce proteins for vaccine constructs, are prokaryotic, and lack much of the complexity of the eukaryotic cell. The bacteria are not able to process proteins in the same fashion that eukaryotes can, making it difficult to develop a properly folded and processed malaria protein in a bacterial vector. If the proteins produced by the bacteria are not identical to the proteins produced by malaria parasites, they will not be recognized by the immune system. This has hampered anti-malaria vaccine production. A eukaryotic vector would be a better candidate, as it would have to proper machinery to process the proteins so that they are identical to the malaria-produced proteins.
A team of researchers at the University of California at San Diego have attempted to make an edible vaccine against malaria. They engineered algae to produce a protein from P. falciparum that has strong immunogenicity in mice. In addition, the researchers engineered the algae to produce a protein from Vibrio cholera, which would be fused to the P. falciparum antigen. The V. cholera protein sticks to intestinal cells. The researchers wanted the engineered fusion protein from the algae to be taken up by the intestinal cells and presented to the immune system within the gastrointestinal tract. When mice were fed the freeze dried algae, they produced substantial amounts of IgA antibody against the P. falciparum protein. IgA is an isotype of antibody that is primarily produced by mucosal immune responses, and is effective against pathogens that infect the mucosae.
Malaria, however, is injected into the blood stream via the bite of a mosquito. The malaria parasite first goes to the liver, where it develops into a form that can then infect red blood cells. The IgA antibody produced by the mice in response to the edible algae vaccine would be ineffective against malaria parasites. IgG antibodies, which are produced in the blood stream, are considered to be more effective against malaria infection by many researchers. Learning how to develop the proper antibody response is one of the important aspects of vaccine production. Improper antibody responses would not be effective at preventing infection.
Even though the vaccine construct does not appear suitable for use against malaria, the researchers are hopeful that it can be used against a variety of mucosal diseases. Many viral and bacterial infections are food borne; they enter the host through the digestive tract. In these cases, a mucosal IgA antibody response produced in the gastrointestinal tract would be very beneficial. The edible algae vaccine vector could be used against such food borne pathogens as Salmonella and E. coli.
While it might seem unusual at first to consider using an oral vaccine, which would target immune cells in the digestive tract, against a pathogen that infects blood cells, the concept is not as far out as one might think. The mucosal immune system and the systemic immune system are connected. Food digested in the gastrointestinal tract is transported to the blood in order to be delivered to other cells in the body. The same is true of antigens. Research has previously demonstrated that certain mucosal vaccination routes, such as a nasal vaccine, can be very effective at eliciting strong systemic immune responses. In fact, the researchers in the above study were able to detect blood IgG antibodies against the V. cholera portion of the fusion protein. This demonstrates clearly that the construct can induce systemic immune responses. The researchers, however, are not sure why the mice did not produce IgG antibodies against the malaria protein.
References:
http://www.sciencedaily.com/releases/201...132607.htm
